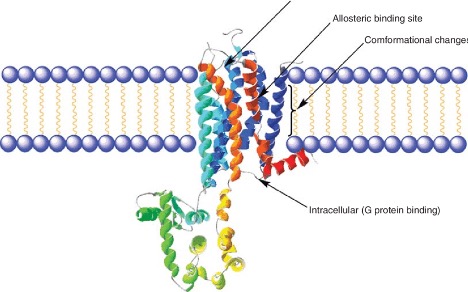
Image: Crystal structure of an adenosine cell surface receptor
We know from previous studies that our brain metabolism can readily use ketone bodies as a primary source of fuel for the brain. What is more, it is widely accepted that ketone bodies (e.g., βHB), are not only a potential energy source for different tissues of the body, but also potent signaling molecules in the central nervous system and systematically [4,5]. We also know that taking exogenous ketones can acutely increase and sustain blood levels of ketone bodies, which is safe and well-tolerated method to reach the state of nutritional ketosis. Because of this, exogenous ketones may be able to alleviate symptoms of different central nervous system diseases by elevating blood ketone levels without having to change dietary macronutrient composition. Indeed, exogenous ketones showed therapeutic potential, for example, in the treatment of anxiety and mental health, different types of seizure disorders and various neurodegenerative disease. However, despite the potential beneficial effects of these molecules in the treatment of central nervous system disorders, their mechanism(s) of action are largely unknown. See our previous post on the benefits and applications of exogenous ketones.
It was suggested that exogenous ketone-induced ketosis can attenuate inflammatory processes, including neuroinflammation, including LPS-induced inflammation.
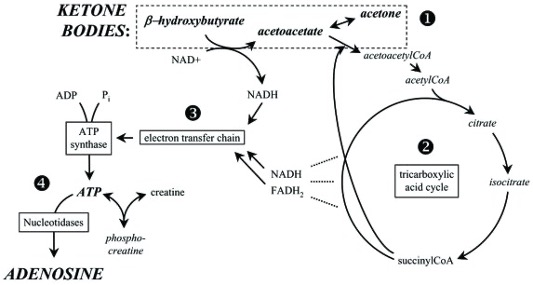
Emerging evidence is implicating the neuroprotective role of adenosine. We know that ketone-induced ATP production can be converted to phosphocreatine for energy storage, or degraded into signaling molecule adenosine. In previous studies it was demonstrated that adenosine is a key molecule as an anti-inflammatory mediator, mainly through A1Rs and A2ARs. It was also suggested that the exogenous ketone supplement-generated alleviatingeffects on absence epileptic activity may be mediated through increased βHB level-evoked increase in adenosine level and A1R activity . Interestingly, activation of A1Rs decreased both the astrocyte proliferation and the excessive activation of microglial cells, thereby attenuating neuroinflammation.
In our latest study we hypothesized that these A1Rs and/or A2ARs cell surface receptors may play a role in the beneficial effects exogenous ketone supplement induce on absence epileptic seizures (reducing the number of seizures) that is caused by a molecule called LPS (in the laboratory, administering this molecule causes the symptoms of epileptic seizures in rodents). Therefore, we investigated how can we influence seizure number if we block the A1R receptor by an A1R antagonist (DPCPX: 1,3-dipropyl-8-cyclopentylxanthine) and the A2AR receptor by an A2AR antagonist (SCH58261: 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine) that we generated by LPS and try to reduce seizure number by exogenous ketone-supplemented food in a rodent model of epilepsy.
We monitored the seizure number by performing EEG recordings on the rodents, while they had unlimited access to exogenous ketone- supplemented food (KEKS food: 10% KE/R,S-1,3-butanediol— acetoacetate diester and 10% KS/Na+ and Ca2+-ketone salt, % by weight, were mixed with powdered standard rodent chow, 1% saccharine and water). We recorded previously that this combination of food increased blood ketone body level and decreased the number of spontaneously developed seizures.
We wanted to investigate the putative effects of combined administration of DPCPX with LPS and SCH58261 with LPS on the seizure number in exogenous ketone-treated animals. We demonstrated earlier that exogenous ketone- containing food alone decreased the seizure number, whereas the receptor blocking antagonists alone did not change the seizure number.
The groups were treated with LPS alone; LPS with A1R antagonist DPCPX (DPCPX + LPS) and an A2AR antagonist SCH58261 (SCH58261 + LPS); and exogenous ketones mixed in the food with LPS (KEKS food + LPS), with DPCPX and LPS (KEKS food + DPCPX + LPS) and SCH58261 and LPS (KEKS food + SCH58261 + LPS).
When DPCPX and LPS were administered combined with ketone supplemented food, the seizure number significantly increased, confirming that the administration of DPCPX abolished the alleviating effect of ketone supplemented food. When SCH58261 and LPS were administered combined with ketone supplemented food there was no change in the beneficial effect of ketone supplemented food.
The total time of seizures increased after the combined administration of DPCPX with LPS, but the total time of seizures did not change significantly after co-administration of SCH58261 with LPS when combined with ketone supplemented food treatment.
With these experiments we further validated our previous results that ketone supplemented food is able to decrease the LPS-induced increase in seizure number and provided new evidence that this alleviating effect of ketone supplemented food may be mediated by the adenosinergic system, likely through A1Rs.
Ketosis may increase the concentration of adenosine; therefore it can modulate the activity of adenosine receptors. It was also suggested that exogenous ketone supplement-generated alleviating effects on absence epileptic activity may be mediated through increased βHB level-evoked increase in adenosine level and A1R activity . Indeed, exogenous ketone supplement-induced increase in βHB level can increase the level of not only extracellular ATP, but also adenosine. It can be an important factor, because adenosine may cause hyperpolarization of neuronal membranes and decrease neuronal activity through, for example, A1R-mediated opening of ATP-sensitive potassium channels and synaptic inhibition resulting in both moderate hyperexcitability in the cortical focus and a decreased seizure number in rodent models. It was also demonstrated that the activation of A2ARs increased the seizure number in rodent models, suggesting that A2ARs are not able to modulate the alleviating influence of exogenous ketone-generated ketosis on the number of spontaneously developed seizures in rodents.
These results suggest that activation of A1Rs and inhibition of A2ARs may evoke alleviating (anti-inflammatory) effects on neuroinflammatory processes. Indeed, activation of A1Rs decreased both the astrocyte proliferation and the excessive activation of microglial cells, thereby attenuating neuroinflammation. It was also suggested that A2ARs can also evoke peripheral anti-inflammatory effects; adenosine may decrease LPS-induced cytokine production through A2ARs.
In summary, our new study that was recently published in the journal Nutrients strengthened the potential of ketogenic supplements, such as exogenous ketone-supplemented food, for the treatment of epilepsy through the inhibition of inflammatory pathways. In relation to the mechanism of action, it is likely that the exogenous ketone-induced ketosis modulated adenosine 1 receptor alleviated the neuroinflammation-induced increase in seizure number.
We showed that inhibition of A1Rs abolished the alleviating effect of exogenous ketone supplemented food on LPS-generated increases in the seizure number, whereas blocking A2ARs did not significantly modify the exogenous ketone supplemented food-generated beneficial effect. Our results suggest that the neuromodulatory benefits of exogenous ketone-supplemented food on absence epileptic activity are mediated primarily through A1R, not A2AR.
This means that theoretically, co-administration of exogenous ketone supplements and different modulators of adenosinergic systems (e.g. adenosine receptors) may allow us to develop promising therapeutic tools in the treatment of not only epilepsy, but also inflammation-evoked neurodegenerative diseases. However, further studies are needed to reveal molecular signaling between exogenous ketone supplement evoked alleviating effects on the seizure number, neuroinflammation, and the adenosinergic system in different cells and brain areas implicated in absence epilepsy genesis.
Written by: Csilla Ari D`Agostino, Ph.D. and Zsolt Kovacs Ph.D
References
Absence seizures. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/conditions-and-diseases/epilepsy/absence-seizures. Accessed December 1, 2021.
Achanta LB, Rae CD. β-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem Res. 2017 Jan;42(1):35-49. doi: 10.1007/s11064-016-2099-2. Epub 2016 Nov 8. PMID: 27826689.
Brunner B, Ari C, D’Agostino DP, Kovács Z. Adenosine Receptors Modulate the Exogenous Ketogenic Supplement-Evoked Alleviating Effect on Lipopolysaccharide-Generated Increase in Absence Epileptic Activity in WAG/Rij Rats. Nutrients. 2021; 13(11):4082. https://doi.org/10.3390/nu13114082
Cahill GF. Fuel metabolism in starvation. Annual Review of Nutrition. 2006;26(1):1-22. doi:10.1146/annurev.nutr.26.061505.111258
Colella M, Zinni M, Pansiot J, et al. Modulation of microglial activation by adenosine A2A receptor in animal models of Perinatal Brain Injury. Frontiers in Neurology. 2018;9. doi:10.3389/fneur.2018.00605
D’Agostino DP, Pilla R, Held HE, Landon CS, Puchowicz M, Brunengraber H, Ari C, Arnold P, Dean JB. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am J Physiol Regul Integr Comp Physiol. 2013 May 15;304(10):R829-36. doi: 10.1152/ajpregu.00506.2012. Epub 2013 Apr 3. PMID: 23552496.
Front Mol Neurosci. 2017 Jul 25;10:235. doi: 10.3389/fnmol.2017.00235. PMID: 28790891; PMCID: PMC5524776.
Kovács Z, D’Agostino DP, Diamond DM, Ari C. Exogenous ketone supplementation decreased the lipopolysaccharide-induced increase in absence epileptic activity in wistar albino Glaxo Rijswijk Rats. Frontiers in Molecular Neuroscience. 2019;12. doi:10.3389/fnmol.2019.00045
Kovács Z, D’Agostino DP, Dobolyi A, Ari C. Adenosine A1 Receptor Antagonism Abolished the Anti-seizure Effects of Exogenous Ketone Supplementation in Wistar Albino Glaxo Rijswijk Rats.
Martí Navia A, Dal Ben D, Lambertucci C, et al. Adenosine Receptors as Neuroinflammation Modulators: Role of A1 Agonists and A2A Antagonists. Cells. 2020;9(7):1739. Published 2020 Jul 21. doi:10.3390/cells9071739
Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014 Jan;25(1):42-52. doi: 10.1016/j.tem.2013.09.002. Epub 2013 Oct 18. PMID: 24140022; PMCID: PMC4176946
Pasquini S, Contri C, Borea PA, Vincenzi F, Varani K. Adenosine and Inflammation: Here, There and Everywhere. International Journal of Molecular Sciences. 2021; 22(14):7685. https://doi.org/10.3390/ijms22147685
Ruskin DN, Kawamura M, Masino SA. Adenosine and Ketogenic Treatments. J Caffeine Adenosine Res. 2020 Sep 1;10(3):104-109. doi: 10.1089/caff.2020.0011. Epub 2020 Sep 16. PMID: 32954218; PMCID: PMC7499891.
van der Putten C, Zuiderwijk-Sick EA, van Straalen L, de Geus ED, Boven LA, Kondova I, IJzerman AP, Bajramovic JJ. Differential expression of adenosine A3 receptors controls adenosine A2A receptor-mediated inhibition of TLR responses in microglia. J Immunol. 2009 Jun 15;182(12):7603-12. doi: 10.4049/jimmunol.0803383. PMID: 19494284.