Despite commonly associated with negative health implications, inflammation is a natural process evolved to protect our bodies from anything that might threaten it. However, when sustained at high levels or inefficiently resolved, inflammation can lead to tissue damage that result in chronic diseases. Keep on reading to learn more about the effects of chronic inflammation in the central nervous system – neuroinflammation – from concepts and mechanisms to how ketogenic strategies may help mitigate it, promoting optimal brain health and longevity.
Acute versus Chronic Inflammation
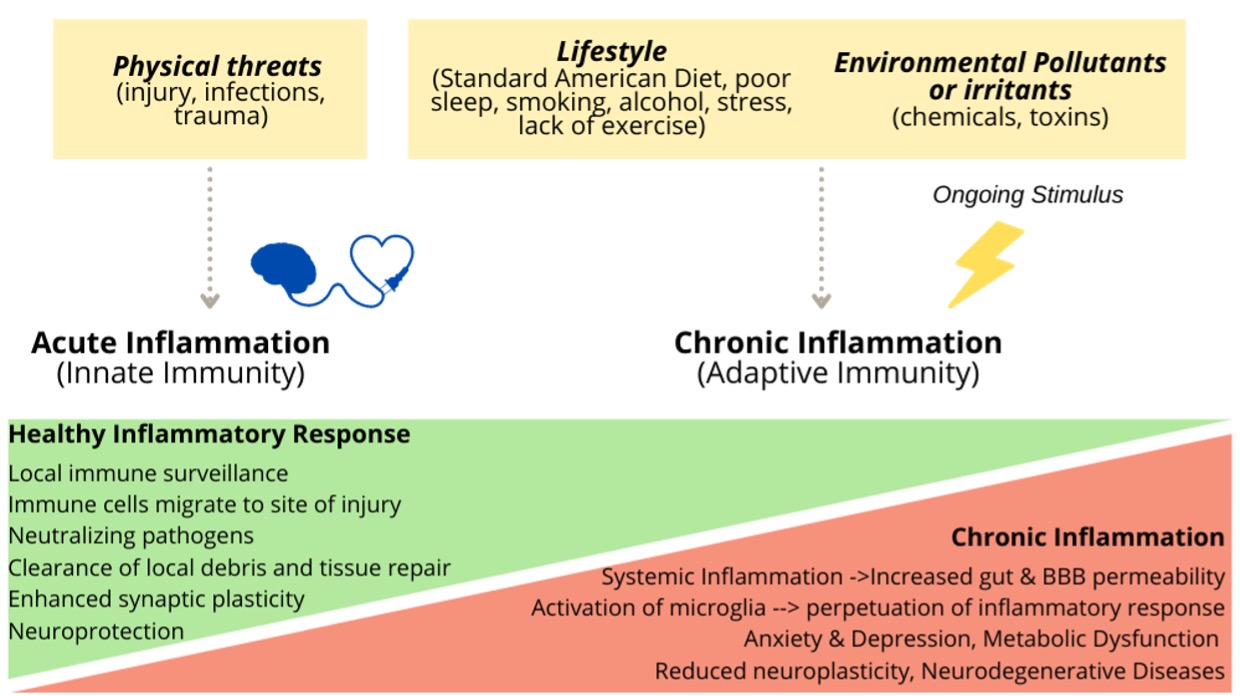
When you suffer an injury, your body activates its defenses to fight off anything that may be endangering its stability. Your immune system releases chemicals that activate an army of white blood cells (leukocytes) to fight infection and promote healing. Acute inflammation (triggered by a paper cut, lifting weights or a cold, for example) usually lasts a few hours or a few days and is essential for tissue repair and restoring homeostasis.
The challenge happens if this immune response lingers, leaving your body in a protracted state of alert and unresolved inflammation. This Chronic inflammation typically results in damage to healthy cells, tissues, organs, and vascular endothelium. Research shows that the proinflammatory state of chronic inflammation is with a cause and consequence metabolic and neurological disorders. [1]
Several prominent and pervasive factors may contribute to triggering and sustaining chronic inflammation, including:
- Infections (bacterial, viral, parasitic, etc.)
- Physical injuries
- Autoimmune conditions (ie, system attacks healthy cells; higher hsCRP, ESR, or ANA))
- Exposure to irritants (toxins in food, on skin or polluted air)[2]
- Lifestyle factors: diet smoking, obesity, alcohol, chronic stress, sleep quality and quantity
Prolonged inflammation in the body can lead to inflammation in the brain
Inflammation can manifest behaviorally as changes in appetite and body weight, fatigue, loss of libido, difficulty concentrating and impaired cognition. Over time, chronic systemic (peripheral) inflammation can result in increased permeability of physical barriers such as the gut lining and the blood brain barrier (BBB), first lines of defense keeping toxic particles and microorganisms away from bloodstream and brain tissue, respectively. Leaky gut may contribute to a leaky BBB, which triggers inflammatory responses within the brain, referred to as neuroinflammation, a complex process that involves the activation of and interplay between several key players:
- Microglia are local immune cells that monitor the environment for insults and are critical to overall brain homeostasis. They remove dead neurons and other cellular debris that may get in the way of neuronal signal transmission. When activated, microglia can produce inflammatory cytokines and chemokines, which facilitate the recruitment of leukocytes to the brain.
- Cytokines are a category of proteins that regulate cell signaling, particularly important when communicating with immune cells. Examples of pro-inflammatory cytokines: interleukins (IL-1β, IL-6) and tumor necrosis factor- α (TNF-α).
- Chemokines are a family of small cytokines known for their ability to stimulate the migration of cells (leukocytes) to sites of infection or tissue damage. Examples: CCL2, CCL5, CXCL1.
- Reactive oxygen species (ROS) refers to an array of chemicals derived from molecular oxygen that are highly reactive (easily bonding to other molecules). Examples: lipid oxidation products, peroxides, superoxide, nitric oxide. Elevated formation and accumulation of ROS leads to molecular damage, defined as ‘oxidative stress’ [3]
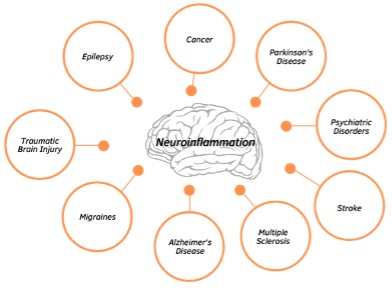
Persistent microglial activation and the production of pro-inflammatory cytokines not only perpetuate microglial activation but also propagate oxidative stress which negatively impacts neuronal mitochondria and BBB permeability. This neuroinflammatory process slows synaptic transmission (communication between neurons) and establish a vicious cycle within the brain that can lead to neuronal death and impaired brain function. In fact, neuroinflammation is considered a pathological hallmark of central nervous system disorders, making it a promising therapeutic target. Several neurological disorders are associated with chronic, low-grade inflammation including depression, migraines, Alzheimer’s disease, schizophrenia, epilepsy, among others.
A commonly used marker to detect inflammation in the body is the C-reactive protein (CRP), a protein released by the liver in response to physical injury. The high-sensitivity C-reactive protein (hs-CRP) is, as the name suggests, a more sensitive and accurate measure of CRP. High CRP levels are highly associated with metabolic dysfunction, obesity, heart disease and neurogenerative diseases, making it an important biomarker to assess metabolic health. [4]
As a general guideline, hs-CRP scores below 1.0 mg/L are correlated with lower inflammatory status. Levels between 1.0 to 3.0 mg/L indicate moderate risk for metabolic and cardiovascular diseases with high risk inferred from readings above 3.0 mg/L. Levels above 10 mg/L usually indicate acute inflammation. [5]
Other markers of chronic inflammation include cytokines and chemokines (measured clinically and in research settings): IL-1β, IL-6, TNF-α, adiponectin (an adipose tissue-derived hormone shown to attenuate inflammation), leptin (also derived from adipose tissue, plays a role in innate immunity), etc. [6] [7]
How can keto help?
Current pharmacological options include nonsteroidal anti-inflammatory drugs (aspirin, ibuprofen, and naproxen) and corticosteroids (suppresses the immune system). Due to potential of side effects from standard pharmaceutical treatments, more people are seeking safe and natural ways to manage inflammation.
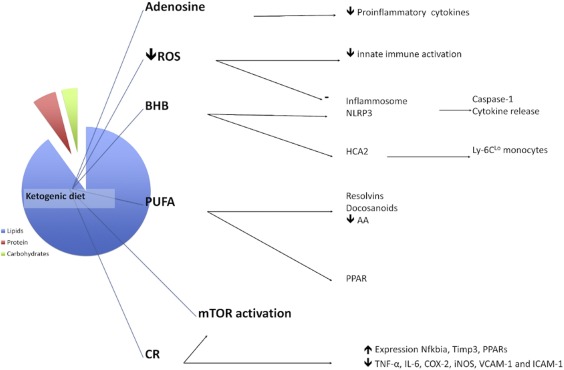
Low-carbohydrate, ketogenic diets (KD) result in increased mobilization and utilization of fatty acids, as a result of low glucose and insulin levels. This metabolic shift results in increased production of ketone bodies in the liver. KDs and ketone bodies have well-established neuroprotective and anticonvulsant properties. Moreover, studies have demonstrated their antioxidant and anti-inflammatory properties. [8] Some of these benefits may indirectly come from reduced consumption of refined carbohydrates (white bread, pastries, sweetened beverages), considered pro-inflammatory. On the other hand, both KD and exogenous ketone supplementation have shown direct neuroprotective and anti-inflammatory benefits in animal and human studies.
- When compared to a low fat diet, KD significantly reduced inflammation in overweight men and women, independent of weight loss, suggesting ketosis-induced improvements metabolic and inflammatory status. [9]
- KDs have been shown to increase brain levels of adenosine, an inhibitory neurotransmitter linked to anxiolytic, anticonvulsant, and anti-inflammatory properties. [10]
- The main ketone body found in the bloodstream, beta-hydroxybutyrate (BHB), can directly inhibit the NLRP3 inflammasome (a major mediator of the inflammatory response) in immune cells, dampening the inflammatory response. [11] This finding has been confirmed in animal models of spinal cord injury and Alzheimer’s disease, showing attenuated neuroinflammation following pre-treatment with either KD or BHB. [12][13]
- Ketone bodies and KDs have been associated with reduction in reactive oxygen species (ROS) and can, therefore, indirectly contribute to lessening of inflammation. [14]
- Our team has published a paper testing the effects of exogenous ketones on an inflammation-induced seizure model. In this study, lipopolysaccharide (LPS – a cell wall component of bacteria) was used to stimulate inflammation and, consequently, epileptic activity. Supplementation with exogenous ketones significantly decreased LPS-induced seizures, likely due to reductions in both neuronal hyperexcitability and inflammation (reviewed in previous blog). [15]
The figure below highlights key mechanisms associated with KD’s anti-inflammatory properties (extracted from [16]).
In addition to ketogenic strategies, other evidence-based methods to consider:
- Lifestyle changes shown to help lower inflammation: Weight loss (if overweight or obese), physical activity, dietary changes, sleep optimization, stress management.
- Diet and Supplementation: Low omega-3/omega-6 ratio (between 1:1 to 4:1), alpha-lipoic acid, curcumin, resveratrol, specialized pro-resolving lipid mediators, exogenous ketone supplements have been linked to decreased inflammation. Specific protocols and dosage will depend on individual, diagnosis, and product specifications.
Main take-aways
Inflammation is a natural part of the immune response, but when it becomes chronic, it should be addressed to attenuate or reverse damage. Recognizing earlier signs of neuroinflammation (brain fog, anxiety, depression, headaches, mental fatigue) and acting as soon as possible may be crucial to dampening and, in some cases, reversing metabolic or neurological impairments associated with chronic inflammation.
Ketogenic strategies like low carbohydrate ketogenic diets or exogenous ketone supplementation are increasingly showing promising anti-inflammatory effects in both pre-clinical and clinical studies. Dietary and supplement strategies are a safe and efficacious metabolic-based approaches to attenuating inflammation, making them an appealing neuroprotective strategy to promote optimal brain health, potentially treat brain injuries, and promote brain longevity.
Citations
| [1] | C. Franceschi and J. Campisi, “Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases,” The Journals of Gerontology, vol. 69, no. Suppl 1, 2014. |
| [2] | M. L. Block and L. Calderon-Garciduenas, “Air Pollution: Mechanisms of Neuroinflammation & CNS Disease,” Trends in Neuroscience, vol. 32, no. 9, pp. 506-516, 2009. |
| [3] | H. Sies and D. P. Jones, “Reactive oxygen species (ROS) as pleiotropic physiological signalling agents,” Nature Reviews Molecular Cell Biology, vol. 21, pp. 363-383, 2020. |
| [4] | Y.-y. Luan and Y.-m. Yao, “The Clinical Significance and Potential Role of C-Reactive Protein in Chronic Inflammatory and Neurodegenerative Diseases,” Frontiers in Immunology, vol. 9, 2018. |
| [5] | P. M. Ridker, J. E. Buring, N. R. Cook and N. Rifai, “C-Reactive Protein, the Metabolic Syndrome, and Risk of Incident Cardiovascular Events,” Circulation, vol. 107, pp. 391-397, 2003. |
| [6] | N. Ouchi and K. Walsh, “Adiponectin as an anti-inflammatory factor,” International Journal of Clinical Chemistry, vol. 380, no. (1-2), pp. 24-30, 2009. |
| [7] | V. Abella, M. Scotece, j. Conde, J. Pino, M. A. Gonzalez-Gay, J. J. Gomez-Reino, A. Mera, F. Lago, R. Gomez and O. Gualillo, “Leptin in the interplay of inflammation, metabolism and immune system disorders,” Nature Reviews Rheumatology, vol. 13, pp. 100-109, 2017. |
| [8] | A. Pinto, A. Bonucci, E. Maggi, M. Corsi and R. Businaro, “Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer’s Disease,” Antioxidants, vol. 7, no. 5, 2018. |
| [9] | C. E. Forsythe, S. D. Phinney, M. L. Fernandez, E. E. Quann, R. J. Wood, D. M. Bibus, W. J. Kraemer, R. D. Feinman and J. S. Volek, “Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation,” Lipids, vol. 43, no. 1, pp. 65-77, 2008. |
| [10] | G. Hasko and B. Cronstein, “Regulation of Inflammation by Adenosine,” Frontiers in Immunology, vol. 4, 2013. |
| [11] | Y.-H. Youm, K. Y. Nguyen, R. W. Grant, E. L. Goldberg, M. Bodogai, D. Kim, D. D’Agostino, N. Planavsky, C. Lupfer, T. D. Kanneganti, S. Kang, T. L. Horvath, T. M. Fahmy, P. A. Crawford, A. Biragyn, E. Alnemri and V. D. Dixit, “The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease,” Nature Medicine, vol. 21, no. 3, pp. 263-269, 2015. |
| [12] | G. Kong, J. Liu, J. Lin, Z. Huang, Z. Yang, X. Wu, Z. Huang, Q. Zhu and X. Wu, “Ketone Metabolite β-Hydroxybutyrate Ameliorates Inflammation After Spinal Cord Injury by Inhibiting the NLRP3 Inflammasome,” Neurochemical Research, vol. 46, no. 2, pp. 213-229, 2021. |
| [13] | D. C. Shippy, C. Wilhelm, P. A. Viharkumar, T. J. Raife and T. K. Ulland, “β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology,” Journal of Neuroinflammation, vol. 17, no. 1, 2020. |
| [14] | M. Maalouf, P. G. Sullivan, L. Davis, D. Y. Kim and J. M. Rho, “Ketones inhibit mitochondrial production of Reactive Oxygen Species production following glutamate excitotoxicity by increasing NADH oxidation,” Neuroscience, vol. 145, no. 1, pp. 256-264, 2007. |
| [15] | Z. Kovacs, D. P. D’Agostino, D. M. Diamond and C. Ari, “Exogenous Ketone Supplementation Decreased the Lipopolysaccharide-Induced Increase in Absence Epileptic Activity in Wistar Albino Glaxo Rijswijk Rats,” Frontiers in Molecular Neuroscience, 2019. |
| [16] | S. Koh, N. Dupuis and S. Auvin, “Ketogenic diet and Neuroinflammation,” Epilepsy REsearch, vol. 167, 2020. |