We recently read Naomi Whittel’s book, Glow15, which we highly recommend for anyone wanting to learn more about autophagy and lifestyle changes for anti-aging.
We recently read Naomi Whittel’s book, Glow15, which we highly recommend for anyone wanting to learn more about autophagy and lifestyle changes for anti-aging.
The book piqued our interest in autophagy and so we started looking into it ourselves.
What we have learned is that there really is no surface level understanding of how autophagy is regulated and this diagram can easily attest to that:
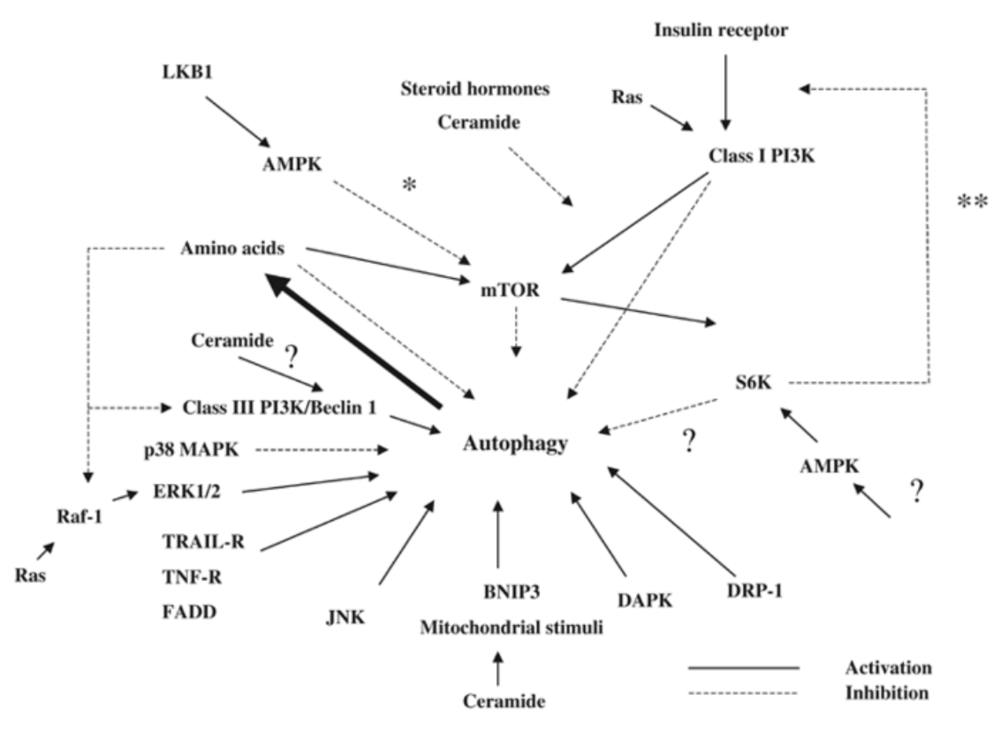
Source: Meijer AJ & Codogno P. 2005. Autophagy
and signaling: their role in cell survival and cell death. Cell Death and Differentiation, 12:1509-1518.
BUT, we at least want to give you a basic understanding of how the process works and touch on some promising areas of research that may translate into practical use.
What is Autophagy:
The word “autophagy” is Greek for “self-eating”, and to no surprise that is its function. It’s a normal mechanism inside each and every one our cells where proteins or whole organelles, that are damaged or just not serving us anymore, are broken down and any individual components salvaged in the process are recycled as a source of energy or to make new cellular structures. The process is always running on a basal level to ensure the quality and survival of our cells. It’s the cellular equivalent to “taking out the trash” so to speak, the only difference is that if we don’t take out the trash we still go on living our lives, whereas our cells do not. Autophagy can be both, pro-survival and pro-death, so it is important that we don’t hold it on a pedestal and dedicate our lives to having it activated at all times.
Take home message: Autophagy is the cellular process that ensures protein and organelle turnover by degrading what is no longer needed and recycling materials to be used again by the cell, either as energy or synthesis of new structures.
Picture your cell as your kitchen
Naomi uses a great analogy in her book comparing the cellular environment to your kitchen. See when you’re working hard to cook dinner, messes get left behind, things drop on the floor, dishes pile up in the sink, and that’s normal, no issues there. Our cells are basically cooking dinner all the time, working hard to perform all their functions, but instead of dishes piling up and messes on the counter tops, damaged proteins start to accumulate and organelles start losing their spunk. After dinner, the last thing we want to do is clean up, but we do it anyways because a clean kitchen makes things run smoothly when the next meal time rolls around. Our cells are doing the same, cleaning up after the messes they’ve made by getting rid of these damaged or dysfunctional components to make sure things can continue to run smoothly. Problems occur when this process slows, and these messes don’t get cleaned up properly, leading to the accumulation of cellular garbage ultimately interfering with our health.
A step back into high school biology
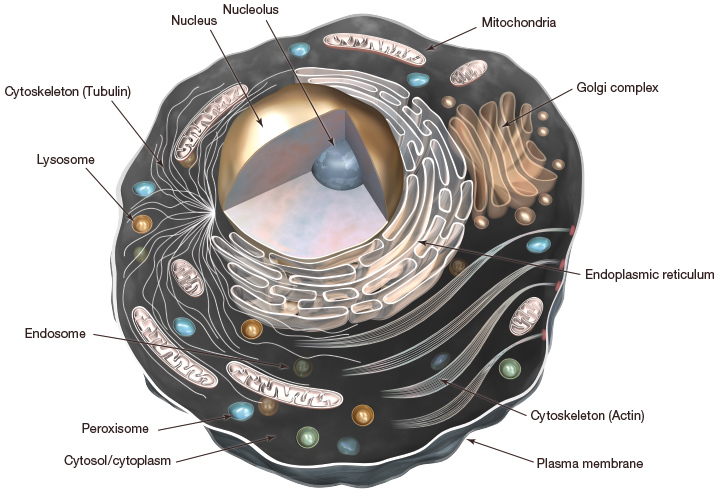
Our cells contain a nucleus and a variety of membrane-bound organelles all enclosed by what’s called the plasma membrane. Different organelles carry out different functions but there is one that we are particularly interested in on the topic of autophagy, and that is the lysosome.
Lysosomes contain all the enzymes needed to breakdown proteins, nucleic acids, carbohydrates and lipids, some people like to refer to the lysosome as the stomach of the cell. Not only do they contain the enzymes needed to breakdown cellular waste, but their membranes contain the transport proteins needed to recycle anything that can be reused by the cell. So, the lysosome is like a little recycling plant, taking in useless material, breaking it down and returning the useable components. This is an important action in autophagy, because not only can we remove these damaged proteins or structures but we provide the building blocks to make new and better ones!
Take home message: The lysosome is the organelle responsible for autophagy and contains degradative enzymes to break down cellular waste (aka the stomach of the cell).
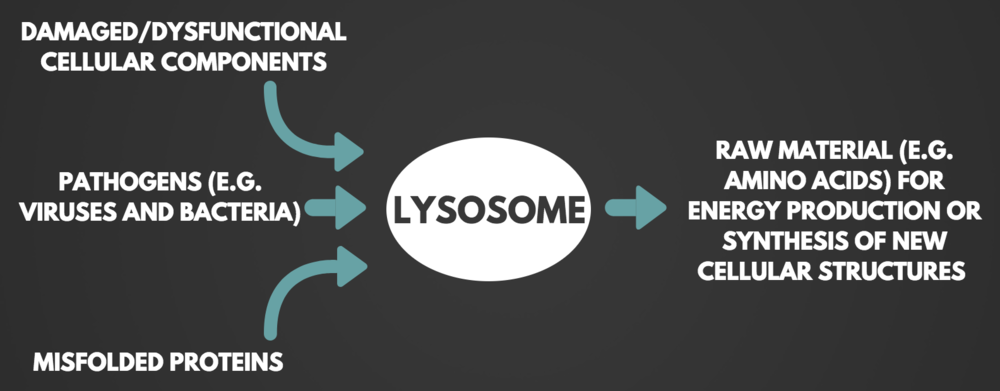
Autophagy at the Cellular Level
In the cytoplasm, the proteins and organelles that are ready to be removed, are sequestered into a double membraned vesicle, called the autophagosome. The autophagosome is delivered to the lysosome where the membranes fuse, making one large compartment, exposing the materials to be degraded to the lysosomal enzymes.
Brief History of Autophagy
Scientists knew early on that our cells had some sort of degradation system after observing cellular content, and even whole organelles, inside the lysosome. It wasn’t until Yoshinori Ohsumi, a Japanese biologist, ran a series of experiments in yeast cells that lacked the enzymes needed to digest these materials in order to observe the buildup of autophagosomes. He then induced genetic mutations, and tested multiple cell lines to identify which genes were responsible for the process of autophagy in which he was successful [1]. This won him the 2016 Nobel Prize in Physiology or Medicine. Scientists are building on Ohsumi’s research and uncovering more on what genes, and their respective proteins, are governing the process of autophagy with attention on its role in health and disease.
Autophagy’s 4 primary roles:
Housekeeping: This is autophagy at a basal level, ridding the cells of damaged or misfolded proteins that may be hanging around and the same for damaged or dysfunctional organelles.
Immunity: Autophagy is a host defence mechanisms, meaning it helps rid the body of pathogenic foreign invaders such as bacteria, viruses, and toxins.
Stress response: Autophagy is triggered in face of cellular stresses such as nutrient deprivation where, in this case, it will breakdown its own cellular components for energy (hint hint… fasting). In the stress response, autophagy is also a cytoprotective (cyto = cell) mechanism to prevent the accumulation of damaged proteins and organelles that could be causing even more harm to the cell.
Embryonic development and cell differentiation: Autophagy plays a role where major tissue remodelling processes are taking place, such as creating a baby!
Why is Autophagy important?
When autophagy stops working, things can go downhill very quickly. Think of the kitchen scenario again, if we just stopped cleaning our kitchens, eventually it would become a massive garbage dump, foods will rot and the place will start to smell. Now picture the inside of our cells with piles of misfolded proteins, and aged organelles, such as old mitochondria, releasing damaging molecules. When misfolded proteins are not cleared from our cells, they form aggregates and lead to the development of amyloids. Amyloids are found in the brain of patience with Alzheimer’s, Parkinson’s, in the arteries of diabetics, and other amyloid diseases. Autophagy is the mechanism that prevents this from happening and autophagy deficiencies have been linked to accelerated aging. Unfortunately, autophagy slows as we age, and this is likely connected to practically all age-related diseases, with emphasis on neurodegenerative diseases. It is therefore important for us to understand how we can optimize this mechanism, turn it on in times of need, and possibly use it as another tool to prevent or mitigate some of the effects of such diseases.
Take home message: Autophagy is essential for the health of our cells and deficient autophagy has been linked to various age-related diseases, due to the inability to clear damaged cellular components that ultimately contribute to disease progression. Autophagy slows as we age, but we may have the power to control it, and that’s why it’s an important topic.
The Mitochondria and “Mitophagy”
There are organelle specific autophagy pathways and they are conveniently named after the organelle at hand. Mitochondrial specific autophagy is referred to as mitophagy. The mitochondria are commonly called the powerhouse of the cell, and if you landed on this page because of your interest in the ketogenic diet, you may already know how incredibly sacred the mitochondria are to our cells.
As with anything that is overused, things start to age and breakdown, and the mitochondria are no exception. The issue is however, that having damaged mitochondria hanging around our cells can be very detrimental. Not only is our energy supply insufficient, but damaged mitochondria increase their production of reactive oxygen species (ROS), and release harmful inflammatory cytokines, both in which wreak havoc on our bodies. In fact, mitochondrial dysfunction is characteristic of practically all metabolic diseases such as diabetes, Parkinson’s, Alzheimer’s, cancer, and many more. Why autophagy, or mitophagy in this case, is such an important preventative and anti-aging mechanism may be becoming evident right about now. It is suggested that mitophagy is regulated by the generation of ROS as a signal to the cell that the mitochondrion is defective and it needs to be replaced [2]. As we age, two major issues arise: autophagy slows and mitochondria are damaged at an increased rate, so if you’re entering your later stages in life, autophagy becomes even more important. Through increased mitophagy, aging may decline at a slower rate and the onset of age-related disease could be delayed.
Take home message: Removing old/damaged mitochondria and replacing them with newer/better is crucial to the health of our cells because they release damaging molecules that contribute to many age-related diseases.
What is Controlling Autophagy?
The initial figure in this post already demonstrated the sheer madness when it comes to the regulation of autophagy, and it is something scientists are still working on. What we do know, however, is that deficient autophagy = shorter life span and that calorie restriction = increased life span, in a range of organisms from yeast to mammals. Autophagy has also been shown to be a required for the life-extension effects of calorie restriction [3]. During periods of starvation, autophagy is activated to provide starving cells with primarily amino acids, to be used for the synthesis of new proteins and other molecules, but also as an energy source by undergoing gluconeogenesis (conversion to glucose).
Calorie restriction and fasting downregulate the insulin/IGF-1 (Insulin-like Growth Factor) and mTOR (mammalian Target of Rapamycin) pathways, both in which have been linked to autophagy. Low insulin levels induce autophagy and high levels, you guessed it, suppress it. That is not the whole story though. A downstream target of insulin is mTOR, which is considered one of the primary regulators of autophagy [4]. mTOR is activated in response to growth factors, amino acids (e.g. dietary protein), and energy status of the cell, so this makes sense why calorie restriction would activate autophagy. Since both of these pathways, insulin/IGF-1 and mTOR, are downregulated during fasting or calorie restriction, it is through their inhibition that autophagy can be activated. There is undoubtedly more to the story which involves AMPK, Beclin-1, tissue-specific regulation, and many other players, but for the sake of your time and your sanity we are going to leave it at that.
Take home message: Autophagy is a multi-regulated process, so there is no one pathway that turns it on or off. Calorie restriction has been proven to activate autophagy, and it is believed that the downregulation of insulin/IGF-1 and mTOR pathways are playing a major role in this.
What You Can Do About It
Fasting
Fasting is a very cut and dry approach to activate autophagy, there’s no questioning which foods to eat and what to abstain from to keep these inhibitory pathways at bay. So, in some ways, fasting is the easiest approach, although I’m sure easy is not what you were thinking since generally speaking, no food = no fun. Multiple studies have demonstrated the neuroprotective effects of calorie restriction and fasting as means of removing toxic molecules and damaged mitochondria from neurons [5], and autophagy is the key player. In one particular study using an animal model, autophagy was upregulated after a 24 hour fast, and results were even more dramatic after 48 hours [6]. With that being said, this was done in a rat model, and fasting hours cannot directly translate to humans, so this information is more informative than practical. They also did not test prior to 24 hours, so it is unknown after what amount of hours autophagy begins activation. The “minimum dose required” would be great to know, because I’m sure we can all agree that reaping the benefits of a 24 hour fast in something closer to 12 hours sounds a lot better than going an entire day, or maybe 3, without eating. As of right now prolong fasts (~3-5 days) are likely what is needed to really enhance autophagy. We believe the research on that forefront will soon be available, due to the current interest in autophagy.
If we are only focused on insulin and mTOR then intermittent fasting is a more realistic strategy. The most common forms of intermittent fasting studied include alternate-day fasting and time restricted feeding, where you alternate between eating normally one day and restrict to ~500 calories or less the next, or eat all of your daily calories in a 6-8 hour window and fast the remaining, respectively. These methods may have some benefit on autophagy by giving our bodies periods of time without food and therefore downregulating our nutrient sensing pathways that, when activated, inhibit autophagy.
Take home message: It is clear that fasting activates autophagy in animal models, it is unclear however how long humans need to fast to activate autophagy. ~3-5 days likely guarantees some autophagy action, but prolong fasting is not for everyone and intermittent fasting may be an alternative.
Ketogenic Diet
Can we induce autophagy without starving ourselves?
Of course, fasting is the simplest way to turn off these nutrient sensing pathways, but fasting is not for everyone (although you could argue, periodic fasting should be for everyone). Nonetheless, a lot of the physiological responses of a ketogenic diet mimic fasting and the reduction in insulin that accompanies the diet and sequential downstream signalling is likely, in part, responsible. In an animal model, the ketogenic diet was shown to upregulate autophagy in the brain and showed a reduction in the release of the highly inflammatory molecule, cytochrome C [8], eluting to the neuroprotective mechanisms of the ketogenic diet. If done properly, the ketogenic diet will induce the metabolic state of ketosis where blood ketones are elevated (> 0.5 mmol/L). Beta-hydroxybutyrate, the primary ketone body, has been shown to stimulate chaperone-mediated autophagy (a type of autophagy that does not require the formation of the autophagosome) in vitro, however this was in the context of nutrient deprivation [9]. There is plenty of other research demonstrating the neuroprotective effects of the ketogenic diet, and how the diet could replace the need for fasting and this could be tied to the activation of autophagy. Thus, we believe the ketogenic diet could be used to, at least moderately, induce autophagy.
Take home message: The ketogenic diet mimics fasting in a lot of ways, and has been shown to induce autophagy in animal models. It is a promising alternative to fasting.
Better Yet, Combine the Two!
Using one of the two intermittent fasting protocols mentioned above, in combination with the ketogenic diet is most likely the best alternative to fasting cold turkey. The reduction in insulin signalling and other nutrient sensing pathways during these periodic fasts in combination with the ketogenic diet, may activate autophagy better than what they may individually, but this is based on speculation and more human data is needed!
Sauna
Some studies have suggested that heat stress such as that associated with sauna use can activate autophagy, since autophagy is a stress response in and of itself. Research in C. elegans (worms), a commonly used model organism, demonstrated the use of heat stress to induce autophagy and extend life-span. Worms, deficient in autophagy did not experience the beneficial effects of the heat stress, indicating that autophagy was responsible for these results. In this study, using a worm model of Huntington’s disease, a reduction of protein aggregation in the brain was observed in, which as previously mentioned, contributes to other neurodegenerative diseases such as Alzheimer’s and Parkinson’s [10]. So, next time you hit the gym, maybe stick around an extra 20 minutes and treat yourself to a sauna session.
Take home message: Mild heat stress could be used as a way to activate autophagy and in worms was shown to reduce toxic protein aggregation in the brain.
Exercise
Speaking of hitting the gym, exercise has also been shown to induce autophagy, and fasted exercise may be even more beneficial. In a mouse model, it was shown that treadmill exercise induced autophagy in the brain [11]. Exercise-induced AMPK and sirtuin 1 could be responsible for these effects, as both are linked to the activation of autophagy, however as always, more research is needed! The many benefits of exercise are actually in response to acute stressors, and this is likely how autophagy is working in this regard.
Conclusion
Autophagy is not as simple as we’d like it to be, but we think with the current understanding of how it is regulated, there are life-style practices that at least wouldn’t hurt to try. The caveat to all of this is that we don’t want these processes on or off at all times and we certainly don’t always want to be in a catabolic state. These growth factors that inhibit autophagy are also what allow us to increase muscle mass, heal our wounds, and regulate many cellular processes for growth and development. It’s also important to recognize that most of this research is done in animal models and in terms of the practical implementation not all data is directly translatable to humans. We still believe autophagy can play a significant role in the prevention and management of many age-related diseases and that knowing how to activate autophagy is another tool in our tool box, just how we think of the ketogenic diet is as well. All in all, autophagy is an extremely important cellular process that is absolutely required for the health of our cells and should be given close attention as we age. Hopefully this article breaks down everything you wanted to know about this hot topic and your leaving with some actionable tips on using temporary stressors to activate autophagy. Now go eat yourself!
Written by: Kristi Storoschuk; Edited by: Dr. Dominic DAgostino
References:
1. Tsukada M, and Ohsumi, Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333(1-2):169-174.
2. Zhang J. Autophagy and mitophagy in cellular damage control. Redox Biology. 2013;1:19-23.
3. Moselli, et al. Calorie restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1(1):e10.
4. Naito T, Kuma, A, and Mizushima N. Differential contribution of insulin and amino acids to the mTORC1-autophagy pathway in the liver and muscle. J Biol Chem. 2013;288:21074-21081.
5. Jaeger PA, Wyss-Coray T. All-you-can-eat: autophagy in neurodegeneration and neuroprotection. Molecular Neurodegeneration. 2009;4:16.
6. Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6(6):702-710.
7. Wang et al. Ketogenic diet attenuates neuronal injury via autophagy and mitochondrial pathways in pentylenetetrazol-kindled seizures. Brain Res. 2018;1678:106-115.
8. Finn PF and Dice JF. Ketone bodies stimulate chaperone-mediated autophagy. J Biol Chem. 2005;280(27):25864-25870.
9. Kumsta C, Chang JT, Schmalz J, and Hansen M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nature Communications. 2017;8:14337.
10. He C, Sumpter, Jr. R, Levine B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy. 2012;8(10):1548-1551.