A gut feeling: Why your stomach hurts when you’re nervous
Imagine the scenario: you are about to present an important talk on a stage with big, bright lights and lots of people in the audience. Suddenly, moments before you step on stage, you get nervous. We’ve all been there- despite months of preparation and hours of practicing; nerves get the best of you as you start to think of everything that could potentially go wrong. What if you forget what you planned to say? What if you trip as you walk on stage?
About 2 minutes later, you notice your stomach gurgle. You may burp or your stomach may clench. Before you know it, you’ve got to run to the bathroom.
That nerve-wracking experience is one we are all too familiar with. Although it seems like mental nerves are unrelated to that trip to the bathroom, those butterflies we feel are actually the most intuitive way to describe the Gut-Brain Axis (GBA), a bi-directional communication system between 1) the brain and 2) the microbiome of the gastrointestinal (GI) tract.
Since the Human Genome Project was published in 2007,1 a new wave of research on the gut microbiome has been ushered in. The gut microbiome is a highly complex ecosystem, comprised of bacterial and genetic components. Scientists are chipping away at our understanding of the gut’s composition and function daily. With each new insight comes the clarity that the gut microbiome is more than just a microbial environment responsible for digesting and absorbing food. More than that, the gut microbiome governs the immune system, synthesizes key neurotransmitters involved in neurologic function, and regulates metabolic responses.
The GBA has recently been attributed to many pathological processes across the spectrum of health. On one end of the health spectrum, for instance, the GBA has been shown to impact anxiety or depression,2 Alzheimer’s disease,3 as well as metabolic dysfunction (i.e., cancer or type II diabetes) and immune-related conditions (i.e., Crohn’s disease and Hashimoto’s thyroiditis).4 On the other end of the spectrum, the GBA has also been shown to influence athletic performance, and those with higher levels of fitness appear to have improved gut function.5
What exactly is the gut-brain axis?
The GBA is a physiologic highway between the central nervous system (CNS) and the enteric nervous system (ENS) that resides along the intestines.6 The ENS is a part of the autonomic nervous system, which carries out essential bodily functions that we don’t have to consciously think about, like breathing, our hearts beating, and digesting food. The GBA is physically connected via the vagus nerve, one of the body’s largest and most influential nerves responsible for parasympathetic activity. The parasympathetic nervous system carries out ‘rest and digest’ functions,7 so it only makes sense that the vagus nerve would be connected to the intestines, the key digestion organ. Most intuitively, the GBA governs the nervous system responses that we have throughout the day. The vagus nerve communicates the needs of the brain to peripheral portions of the nervous system, like the ENS. In turn, the ENS along the gut microbiome produces many of the metabolic, immune, and neurologic constituents that the brain then uses for neurologic functions.2 Thus, reductions in activity of the vagus nerve can contribute to GI distress, like irritable bowel symptoms or constipation.
The GBA influences other neurologic systems, including the hypothalamic-pituitary-adrenal (HPA) axis, which governs stress responses.8 Imagine you’re on that big stage about to present- your stress response is upregulated via the sympathetic nervous system (i.e., ‘fight or flight’ mechanism), and stress hormones are secreted, inhibiting vagal tone and reducing GI functions. In contrast, beneficial gut bacteria have been shown to reduce stress hormones and promote vagal activity. What’s even more interesting is that the gut microbiome actually synthesizes key neurotransmitters involved in mood and stress responses, including serotonin and dopamine. In fact, 70-80% of dopamine and upwards of 90% of serotonin are synthesized by way of the gut microbiome!9,10 The GBA can also regulate immune functions by responding to external stressors (i.e., pathogens or endotoxins) that the gut microbiome is exposed to. Metabolically, the gut microbiome utilizes constituents from digestion (i.e., short-chain fatty acids or amino acids) to carry out key functions that translate to the nervous and immune systems.
In turn, these immune, neurologic, and metabolic constituents traverse the brain by way of the GBA to regulate neurologic function. This results in a relatively stable equilibrium between the gut microbiome and the brain. But, if the gut microbiome is dysregulated,11 the three roles of the gut microbiota are specifically altered in the following ways: 1) increased environmental stressors upregulate gut inflammation leading to an excessive immune response in which the GBA functions are altered; 2) impaired ENS from this damage inhibits neurotransmitter signaling; and 3) dysregulated metabolic function due to an unfavorable alteration in bacterial and metabolite concentrations.4
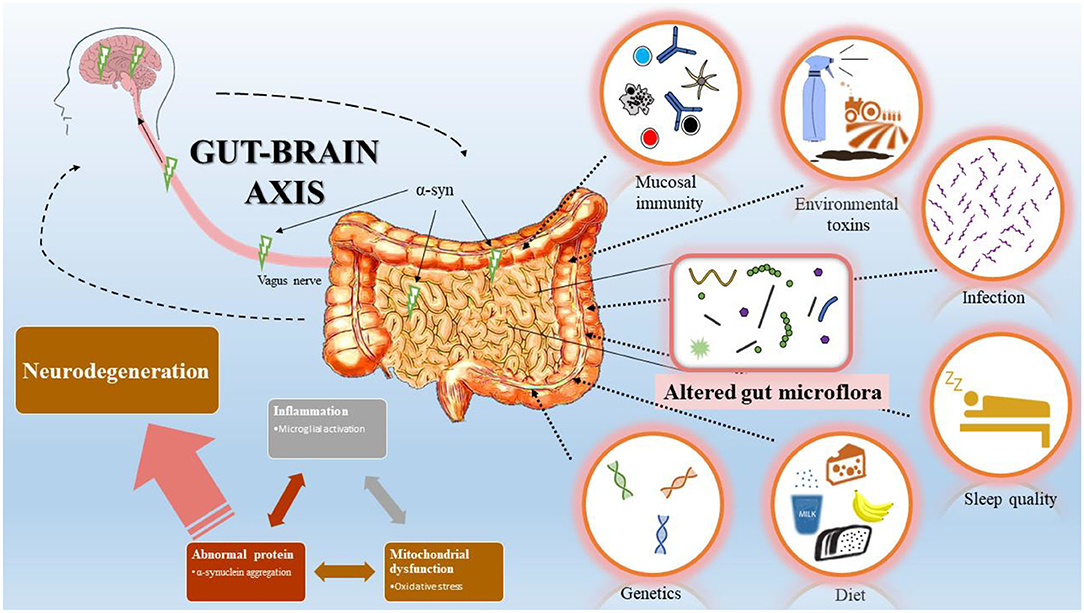
What we know in gut-brain axis research
1) The GBA can be improved by modulating the vagus nerve
The vagus nerve is critically important, and vagal dysregulations have been shown to affect GBA communication. This disruption to the GBA is attributed to mood disorders, immune-related conditions, sleep health, and many chronic diseases! Promising research has come out of the gut microbiome space to show that modulating the vagus nerve positively impacts overall GBA function.
- Breathwork that is often done in meditation or mindful exercise has been shown to increase the parasympathetic responses of the vagus nerve to improve mood and digestion.12
- Cold exposure- like taking a cold shower or sitting in an ice bath for a few minutes- can inhibit an overactive sympathetic nervous system to help increase vagal tone.13
- Exercise that promotes a mind-body connection, like yoga14 or Tai Chi,15 can also improve vagal tone.
- Diets, like those low in carbs, have also been shown to significantly improve vagal nerve function, particularly in those living with epilepsy.16
2) The gut is influenced by diet, including foods that promote digestion and neural function
Psychobiotics, or external factors that interact with the gut microbiome,17 impact gut health. One common psychobiotic is food. Of course, the first thing that comes to mind when we think of the gut microbiome is FOOD. What and how much we eat matters as food impacts the gut microbiome. Certain dietary patterns promote beneficial bacterial strains to be present in the gut, which can significantly affect gut health and, thus, improve GBA communication. There are many factors to consider when it comes to determining the best diet to improve gut health. However, the gut microbiome research community has come to a few consensuses. For one, a Western-style diet- full of processed, fried, sugary foods- has a negative impact on the gut microbiome. However, conflicting evidence exists as to what type of diet is best to improve vagal function and overall gut health.18 Both high-fat and high-sugar diets have been shown to influence vagal nerve function to improve the connection between the gut and brain.19 In any case, it seems as though eating foods rich in prebiotic, fiber-rich foods and probiotics can help the gut microbiome function better, meaning reduced irritable bowel symptoms20 and reductions in constipation.21
3) The gut is influenced by exercise

One super interesting area of research that has gained popularity in the last decade is that exercise that improves aerobic and muscular fitness can not only regulate vagal tone but also promote the production of beneficial gut markers. In 2014, Clark et al. was the first to show that athletes with increased fitness levels have a more diverse gut microbiome, independent of many dietary conditions.22 Since then, numerous studies have confirmed a relationship between markers of gut health and fitness levels, motivating exercise intervention studies to restore gut health.23
Ketones and the Gut-Brain axis
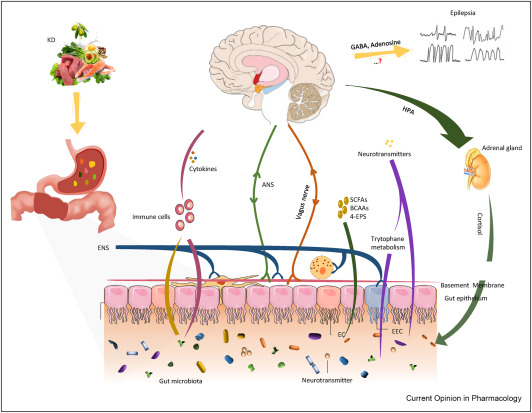
Source: Tang et al., 2021. Microbiota-gut-brain axis: A novel potential target of ketogenic diet for epilepsy. Current Opinion in Pharmacology, 61:36-41.
Emerging research suggests that the influence of ketogenic diets on the gut microbiome contribute to the therapeutic efficacy of the diet, and in particular, epilepsy. Under states of nutritional ketosis, plasma ketone bodies (beta-hydroxybutyrate and acetoacetate) are elevated and exert both systemic metabolic and signaling effects. Ketone bodies become a major fuel source for the brain, and can change neurotransmitter levels such as increasing GABA (our calming neurotransmitter), reducing glutamate (our excitatory neurotransmitter), and restoring adenosine levels and it is thought that these changes contribute to the neurological benefits of the diet. But, what does this have to do with the gut? Interestingly, in a mouse model of epilepsy, the anti-epileptic success of the diet was associated with an increase in two particular gut bacteria strains. When these strains were isolated and administered to mice on a standard diet, the anti-epileptic effect of the ketogenic diet was restored suggesting that the changes on the gut microbiome were responsible for the success of the ketogenic diet. This also suggests a clear cross-talk between the gut and the brain that was influenced by the ketogenic diet. In children with epilepsy, the ketogenic diet-induced changes to the gut microbiome have also been connected to the regulation of immune factors implicated in seizure control. In microbiome research, high-fat diets are typically regarded as pathological in terms of their effects on the gut microbiome, but a recent study demonstrated that the gut microbiota of mice on a high-fat diet (typical of a Western/Standard American Diet) is distinct from that of a high-fat ketogenic diet. This is very important to recognize in all research where a “high-fat” diet is followed, as this is typically high-fat-high-sugar, producing very different results from a high-fat-very-low-carb ketogenic diet. Collectively, while this evidence is intriguing no doubt, much more research is needed to identify the bidirectional cross-talk between the gut and the brain associated with ketogenic diets and ketone bodies themselves (e.g., exogenously supplied) and how these interactions may contribute to the diets’ therapeutic success not only for epilepsy, but also mental health disorders, neurological disorders, metabolic syndrome, etc.
What we still don’t know about the gut-brain axis
Research about the GBA is a hot topic, and we are discovering more about this bi-directional communication system daily. The evidence that does exist is really exciting- it’s clear that the gut microbiota plays an influential role in many different diseased states, and improving gut health likely will benefit us. Still, despite the exciting findings in the last decade about gut health and the GBA, it’s important to remember that there is still so much unknown about this entity, and misinterpretations are often made about gut health overall.
It’s difficult to clearly define a ‘healthy’ gut microbiome
Since the Human Genome uncovered the gut microbiome, research on gut health has vastly expanded and scientists are continuing to chip away at answering new questions about the highly-complex microbial ecosystem. Early gut research defined gut alterations as dysbiosis, or an imbalance between beneficial bacterial strains and pathogens,11 measured typically as bacterial abundance and diversity (or richness). For instance, it was determined pretty early on that bacteria like Lactobacilli were considered ‘healthy’, whereas bacteria like Escherichia coli were pathogenic. However, perplexing evidence came from this early research, showing that certain bacteria appeared to be ‘beneficial’ in some people but ‘pathogenic’ in others. While bacterial abundance and diversity can capture the composition of the gut, each bacterial strain has more than one function. The ushering in of new methodological advances to measure the gut has allowed us to better understand the microbial landscape. We now know that measuring bacteria only scratches the surface of the gut microbiome.24 It is also imperative to look at ‘functional markers’ of the gut, like inflammatory markers or metabolites. Until more of this evidence is teased out, it is important to proceed with caution when trying to define ‘gut health’ as a way to improve the GBA.
We don’t yet know the exact interventions that are best suited to improve the gut microbiome and GBA
The gut microbiome is still a vast mystery of sorts, and our understanding of the gut changes daily as new advances in science develop. Thus, it is critically important that we take the influence of the GBA with a grain of salt, and tread lightly in regard to how we ‘improve GBA connection’ or ‘restore gut functions’. The most effective diet, exercise, or lifestyle interventions- like breathwork or cold exposure- have yet to be confirmed. Even still, the research that does exist is promising, and it’ll be exciting to see the discoveries that come about in gut research in years to come!
Written by: Kaylie Zapanta. Kaylie Zapanta is currently a doctoral student at USC studying the intersection between exercise physiology and the gut microbiome. In addition to her research she is heavily involved in science writing/editing, public outreach, and mentorship. She is also a certified strength and conditioning specialist, nutrition coach, and personal trainer working with individuals to help improve their health through diet and exercise.
Website: www.healthyfitsaved.com
Instagram and Twitter: @KaylieZapanta
YouTube: KaylieZapanta, or https://youtube.com/channel/UC19Lg3uqO9Cx1oyD2Am_jmA
References:
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804-810.
- Breit S, Kupferberg A, Rogler G, Hasler G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front Psychiatry. 2018;9:44.
- Bostanciklioglu M. The role of gut microbiota in pathogenesis of Alzheimer’s disease. J Appl Microbiol. 2019;127(4):954-967.
- Dinan TG, Cryan JF. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol Clin North Am. 2017;46(1):77-89.
- Marttinen M, Ala-Jaakkola R, Laitila A, Lehtinen MJ. Gut Microbiota, Probiotics and Physical Performance in Athletes and Physically Active Individuals. Nutrients. 2020;12(10).
- Zhu X, Han Y, Du J, Liu R, Jin K, Yi W. Microbiota-gut-brain axis and the central nervous system. Oncotarget. 2017;8(32):53829-53838.
- Browning KN, Verheijden S, Boeckxstaens GE. The Vagus Nerve in Appetite Regulation, Mood, and Intestinal Inflammation. Gastroenterology. 2017;152(4):730-744.
- Hueston CM, Deak T. The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiol Behav. 2014;124:77-91.
- Reigstad CS, Salmonson CE, Rainey JF, 3rd, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29(4):1395-1403.
- Eisenhofer G, Aneman A, Friberg P, et al. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab. 1997;82(11):3864-3871.
- Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191.
- Bordoni B, Purgol S, Bizzarri A, Modica M, Morabito B. The Influence of Breathing on the Central Nervous System. Cureus. 2018;10(6):e2724.
- Jungmann M, Vencatachellum S, Van Ryckeghem D, Vogele C. Effects of Cold Stimulation on Cardiac-Vagal Activation in Healthy Participants: Randomized Controlled Trial. JMIR Form Res. 2018;2(2):e10257.
- Tyagi A, Cohen M. Yoga and heart rate variability: A comprehensive review of the literature. Int J Yoga. 2016;9(2):97-113.
- Hamasaki H. Exercise and gut microbiota: clinical implications for the feasibility of Tai Chi. J Integr Med. 2017;15(4):270-281.
- Song X, Wang L, Liu Y, et al. The gut microbiota-brain axis: Role of the gut microbial metabolites of dietary food in obesity. Food Res Int. 2022;153:110971.
- Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016;39(11):763-781.
- Klingbeil E, de La Serre CB. Microbiota modulation by eating patterns and diet composition: impact on food intake. Am J Physiol Regul Integr Comp Physiol. 2018;315(6):R1254-R1260.
- Jamar G, Ribeiro DA, Pisani LP. High-fat or high-sugar diets as trigger inflammation in the microbiota-gut-brain axis. Crit Rev Food Sci Nutr. 2021;61(5):836-854.
- Simon E, Calinoiu LF, Mitrea L, Vodnar DC. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients. 2021;13(6).
- Dimidi E, Christodoulides S, Scott SM, Whelan K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv Nutr. 2017;8(3):484-494.
- Clarke SF, Murphy EF, O’Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913-1920.
- Clauss M, Gerard P, Mosca A, Leclerc M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front Nutr. 2021;8:637010.
- Chen MX, Wang SY, Kuo CH, Tsai IL. Metabolome analysis for investigating host-gut microbiota interactions. J Formos Med Assoc. 2019;118 Suppl 1:S10-S22.