Ketogenic diets and ketone supplements have become an emerging area of research for the prevention, treatment, and management of several diseasese and disorders. Ketones are produced by the liver under conditions of low glucose and insulin, such as ketogenic diets and fasting, or can be taken as a supplement to induce a state of ketosis independent of diet. Once in the blood, ketones travel to our tissues where they can be used as a source of fuel for our mitochondria as an alternative to glucose.
Mitochondria are little structures inside our cells where various fuels, including ketones, are turned into the energy we need to live, in the form of ATP, our cell’s energy currency. Beyond their metabolic roles, ketones also serve as signaling molecules; altering the expression of genes and interacting with various processes in the body including our immunity, cognition, and oxidative stress pathways, for example. Interestingly, most pathologies that ketogenic therapies benefit have metabolic abnormalities central to the mitochondria. In addition, mitochondrial function is absolutely essential for human health. This begs the question of whether ketones may help improve mitochondrial function.
Source: Vidali et al., 2015
So what do we know about ketones and mitochondria?
Ketones support mitochondrial metabolism and endogenous antioxidant capacity
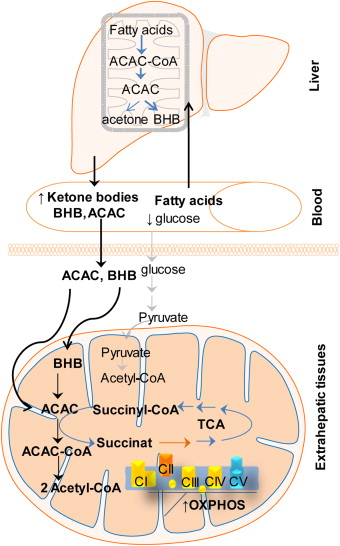
Ketosis is defined as a shift in energy metabolism towards ketone and fatty acid utilization. Early experiments found that when the working rat heart was fed ketones (beta-hydroxybutyrate; BHB), ATP production increased beyond what was produced with glucose. While BHB is our primary ketone body produced during ketogenesis, we also produce acetoacetate (AcAc) and acetone in smaller amounts. However, BHB must first be converted to AcAc before entering mitochondrial metabolism. It was discovered that this conversion altered certain components of the electron transport chain (ETC) in the mitochondria, and this was responsible for the greater generation of ATP.
This shift from glucose to ketone metabolism, and therefore higher demand on our mitochondria has been proposed to elicit changed in mitochondrial function through a process called mitohormesis.
Source: Yang et al., 2019
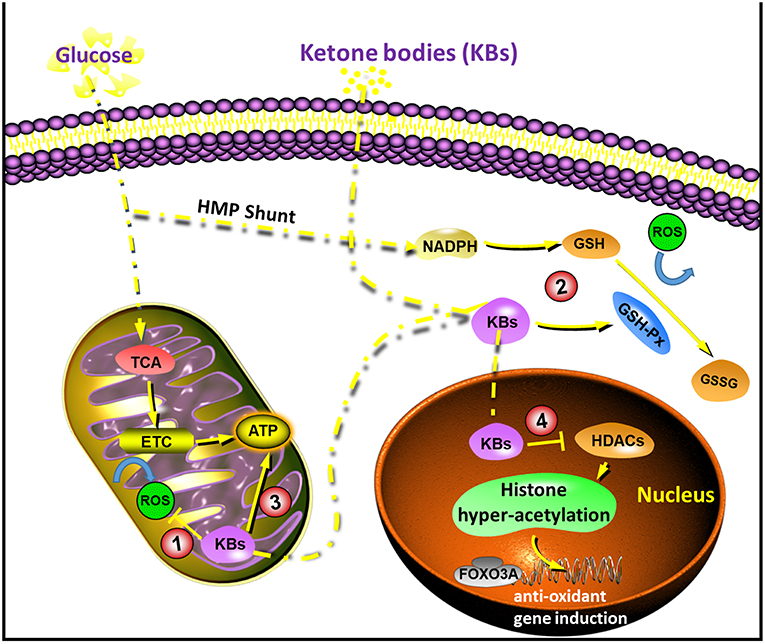
When fuels are burned in the mitochondria, reactive oxygen species, or ROS, are formed. Generally speaking, ROS are viewed as bad guys, because they contribute to oxidative stress and cell damage when they accumulate past our cell’s antioxidant capacity. However, a small increase in ROS is actually beneficial and can trigger adaptations that make us better capable at cleaning up ROS before they cause damage. The initial onset of ketosis in rats has been shown to trigger an acute rise in ROS that our cell’s respond to by strengthening and adapting our antioxidant system in preparation for future stress. One such way is by increasing the synthesis of glutathione, a major player in our cell’s antioxidant system that sweeps up and disarms ROS. Interestingly, in a model of glutamate excitoxicity, a condition known to elevate mitochondrial ROS and wreak havoc on neurons, ketones were protective against glutamate excitotoxicity by improving mitochondrial metabolism and thereby lowering ROS production.
Ketones may improve mitochondrial function via signaling roles

Beyond ketones role as an energy substrate, BHB is also a potent signaling molecule that can beneficially influence the expression of genes and processes in the body. In mice, ketogenic diets upregulate the expression of genes involved in mitochondrial metabolism, in addition to antioxidant genes, both of which support mitochondrial function. Ketogenic diets are also associated with an increase in several components of the electron transport chain and fatty acid metabolism. In fact, rats fed a ketogenic diet show increased mitochondrial content in the brain associated with an enhancement of brain metabolism. By improving brain energy metabolism, this increase in mitochondrial content and function is proposed to be one mechanism for which the ketogenic diet elicits in anti-seizure effects. Brain energy metabolism is a critical component of cognition, and mitochondrial dysfunction can contribute to neurological diseases and cognitive decline. Thus, ketones, either produced through fasting or carbohydrate restriction, or taken as a supplement, may be a means of protecting our brain as we age, through the effects of ketones on our mitochondria. Lastly, in a model of traumatic spinal cord injury, which is associated with impaired brain energy metabolism and mitochondrial dysfunction, a ketogenic diet was neuroprotective by essentially rescuing mitochondrial function.
Ketones may improve mitochondrial function when paired with exercise
Mitochondrial adaptations to exercise are one of the primary benefits of exercise. Higher oxidative capacity (fat metabolism) in our muscle, is associated with greater health, and muscle mitochondrial dysfunction is associated with insulin resistance and type-2 diabetes. Exercise alone, independent of diet, is essential for supporting and promoting muscle mitochondrial function, however, there is emerging evidence that exercising in conjunction with ketosis may alter these mitochondrial adaptation to exercise in a positive way. For example, in a 12-week exercise trial, greater mitochondrial capacity and efficiency was found in the ketogenic diet group compared to the control. In mice, a ketogenic diet with exercise training resulted in an increase in marker of mitochondrial biogenesis (formation of new mitochondria). Future research is required to elucidate potential synergistic effects of both ketogenic diet and exogenous ketones with exercise training on mitochondrial adaptations, as this is a very new, but exciting, area of research.
Conclusion
Altogether, the shift in metabolism associated with being in a state of ketosis increased the demand on our mitochondria. Preliminary research suggests that this increased reliance on mitochondrial energy metabolism may trigger adaptations that improve mitochondrial function. In addition, simply by virtue of how ketones are metabolized by our cells, ketones may be protective against oxidative stress, another hallmark of aging and chronic disease. Given the strong link between several diseases and mitochondrial dysfunction, this is likely one mechanism for which ketones elicit their therapeutic effects.
