At some point, we have all experienced pain and it is the most common reason for scheduling a doctor’s appointment. Pain warns us of potential danger and serves an important biological function. Like inflammation, acute pain is short-lived, and typically a result of a noxious stimuli, potentially leading to an injurious event. Unfortunately, an injury that produces acute pain can progress to chronic pain in some individuals. Progression to chronic pain in some individuals can be physically and mentally debilitating. Chronic pain remains somewhat arbitrarily chronically defined as persisting past 3 months and can also develop gradually, without a specific event. This “idiopathic pain” can be caused by or contribute to mental distress, and is a subject of intense research. Generally speaking, we know that individual variability in pain is a result of genetics, psychological factors, environment and our overall physiological and metabolic health. Most importantly, we can proactively do things to modify our pain tolerance and pain perception.
A 2020 report from the CDC noted that the 2019 National Health Interview Survey found that 20.4% of adults reported chronic pain, while 7.4% had chronic pain that frequently limited life or work activities. Currently, a biopsychosocial model of pain management is being utilized more often in clinics across America. The primary goal of this model is to address, and potentially reverse, the factors that are contributing to pain by offering a wider variety of treatment options. Pharmacotherapy is a component of the biopsychosocial model and attempts to address the molecular factors that may be contributing to chronic nociceptive and/or neuropathic pain. The “Pain ladder”, created by the World Health Organization (WHO), was established as a drug guideline for managing pain.
The “ladder” of pharmacotherapy begins with the use of NSAIDs and then progresses from weak to strong opioids. While there is a place for mindful pharmacological management, it is not without risk. It is well-known that the use of opioids has contributed to a significant societal problem. However, long-term NSAID use also poses significant problems. For example, inhibition of the COX enzymes, responsible for synthesis of the prostaglandins that inhibit acid secretion, can lead to gastric distress. There are also adjuvant analgesics (e.g. Gabapentin, SNRIs, TCA’s that were originally developed for other purposes but have been applied to pain conditions. Unfortunately, these medications can also have significant side effects with long term use.
Moving forward, it is imperative to consider innovative ways to complement traditional pain management. This could include several interventions, but the primary focus here is on the potential role of changing metabolic physiology and signaling through dietary ketosis. There are several intriguing mechanisms that could be responsible for pain relieving affects, including ketone-induced signaling (eg. HDACIs, GPR109A, etc.), and a reduction in glucose, insulin signaling and several inflammatory biomarkers implicated in pain signaling.
Ketogenic Diet and Latency to Pain
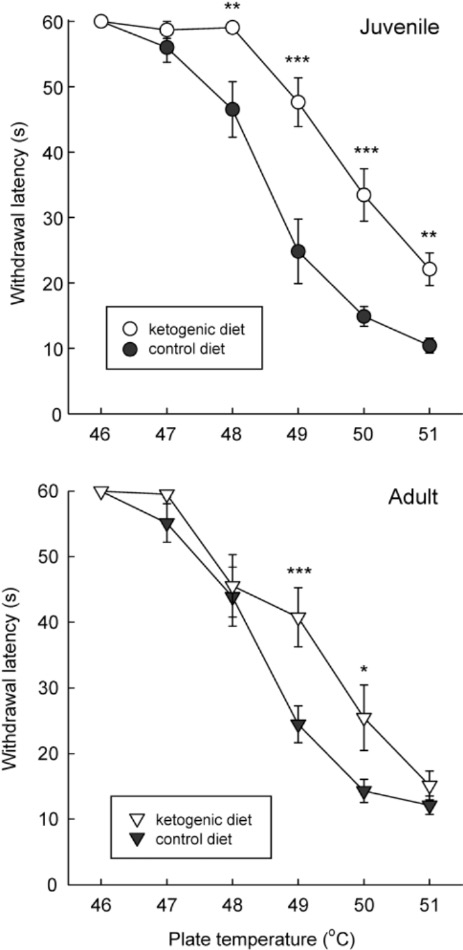
Researchers at Trinity College noted in their review article that they were interested in understanding the relationship of a ketogenic diet and response to thermal pain. In their words, the researchers stated: “rats are placed on a warm plate and their latency to display discomfort (licking or raising their hindpaws) is recorded and the animal is removed immediately.”
Interestingly, they found that rats on a ketogenic diet for several weeks vs. control diet exhibited hypoalgesia to thermal pain (Figure 1). In other words, there was an increased duration of time to show behavioral indications of pain.
In their review article, the researchers also note that additional studies with a more liberal diet produced similar effects. Ultimately, they have demonstrated that metabolic adaptations that occur with a ketogenic diet can influence pain signaling.
Inflammation
The inflammatory response is a complicated phenomenon mediated by the immune system. While the purpose of inflammation is to help maintain homeostasis, chronic inflammation could be implicated in pain conditions. Therefore, a metabolic strategy that could reduce inflammation may provide relief from pain, suggesting a metabolic-induced neural modification of nociception.
Masino and Ruskin discuss several examples in their review article but one that is particularly intriguing is that a ketogenic diet reduced central inflammation in a rodent model of multiple sclerosis. After feeding with a ketogenic diet (KD) versus standard diet (SD), Do Young Kim and colleagues found that the KD mice had favorable changes in immune-cell distribution. Also, they had a “reduction in expression levels of cytokines (IL-1β, IL-6, TNF-α, IL-12, IL-17) and chemokines (IFN-γ, MCP-1, MIP-1α, MIP-1β), compared with SD-fed EAE (rodent MS model) mice in the periphery and in the brain.” A notable point here is that cytokines and chemokines are involved in the production of reactive oxygen species (ROS), which in turn contribute to inflammation. Consequently, by reducing the upstream cause of the production of the ROS, a decrease in inflammation follows.
Another compelling mechanism for reducing inflammation is through modulation of the Nod-like receptor protein-3 (NLRP3) inflammasome pathway. The pathway can be initiated by a variety of molecular and cellular stimuli – a response that is critical for innate immunity but has also been linked to the pathogenesis of inflammatory disorders. Interestingly, there is data emerging that shows aberrant functioning of the NLRP3 inflammasome in pain conditions such as gout pain, post-operative pain, and muscle pain, to name a few. Since data is suggesting this pathway is involved in the pathogenesis of chronic pain, innovative ways to target it are exciting. To that end, a paper published in Nature Medicine in 2015 found that B-hydroxybutyrate (BHB) suppresses NLRP3 activation in some conditions. BHB is one of the ketone bodies produced in a state of ketosis; therefore, following a ketogenic diet may alleviate inflammatory pain attributed to activation of the NLRP3 pathway. The investigators also administered exogenous supplemental ketosis, and demonstrated that the BHB-induced NLRP3 suppression was achieved with both BHB enantiomers (D-BHB and L-BHB). Since L-BHB is metabolized slower and stays elevated longer (partly converting to D-BHB), it is possible that racemic formulations elevating L-DHB may offer greater anti-inflammatory effects, but these studies have not been done.
Psychological Effects of Chronic Pain
An additional component of chronic pain conditions to consider is the effect on well-being. Individuals with chronic pain are more likely to suffer from psychological disorders such as anxiety, depression, and other disturbances. Interestingly, the relationship between chronic pain and psychological disorders seems to be bi-directional, because of shared underlying neurobiology between the two conditions. Several studies have found a ketogenic diet to have positive effects on mental health. For example, a review article published in May 2021 examined articles that assessed the ketogenic diet in various mental health disorders. Overall, the studies they reviewed showed positive results, but the authors do note that additional studies are needed. Because of shared neurobiology, the ketogenic diet may work through similar mechanisms to alleviate pain and improve mental health. More information on the ketogenic diet and mental health can be found in a previous blog.
Non-pharmacological strategies to manage chronic pain
There exists strong scientific rationale for managing pain by improving metabolic and inflammatory biomarkers, which can be achieved through dietary, supplement or exercise interventions. As mentioned, the use of the KD for pain management has been studied in several animal model systems and is now an area of emerging human clinical research (eg. NCT03710798, NCT05085483). Various nutraceuticals are moderately effective for some forms of pain, but the quality of these studies is often lacking and more RCTs are needed. Numerous studies support the observation that pain sensitivity is reduced by exercise training, so this is one tool that should be leveraged. The optimal pain management strategy is likley a synergy of these approaches that maybe be used as a replacement for drug therapy or as multimodal program with pharmacotherapy.
Summary
- Pain is the leading cause for doctor visits.
- Additional, innovative strategies are needed to help manage pain
- A gradual shift in metabolism to ketosis may…
- Dampen pain signaling, inflammation, and other factors contributing to pain
- Provide a benefit for the psychological aspects of pain
- Exercise-induced changes alter pain sensitivity, which may be linked to neurological and metabolic changes.
- Like other therapies, it is important to properly implement the use of a ketogenic diet, so be sure to work with a trusted professional
Written by David J Giordano
davidjgiordano@outlook.com
References
Chronic pain and mental health. Mental Health America. https://www.mhanational.org/chronic-pain-and-mental-health.
Chen R, Yin C, Fang J, Liu B. The NLRP3 inflammasome: an emerging therapeutic target for chronic pain. J Neuroinflammation. 2021;18(1):84. Published 2021 Mar 30. doi:10.1186/s12974-021-02131-0.
Hooten WM. Chronic Pain and Mental Health Disorders: Shared Neural Mechanisms, Epidemiology, and Treatment. Mayo Clin Proc. 2016 Jul;91(7):955-70. doi: 10.1016/j.mayocp.2016.04.029. Epub 2016 Jun 22. PMID: 27344405.
Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2017 Jan;37(1):29-42. doi: 10.1007/s00296-016-3481-8. Epub 2016 Apr 23. PMID: 27107994.
Kim DY, Hao J, Liu R, Turner G, Shi FD, Rho JM. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One. 2012;7(5):e35476. doi: 10.1371/journal.pone.0035476. Epub 2012 May 2. PMID: 22567104; PMCID: PMC3342287.
Masino SA, Ruskin DN. Ketogenic diets and pain. J Child Neurol. 2013;28(8):993-1001. doi:10.1177/0883073813487595.
Products – data briefs – number 390 – November 2020. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/products/databriefs/db390.htm.
Ruskin DN, Kawamura M, Masino SA. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS One. 2009 Dec 23;4(12):e8349. doi: 10.1371/journal.pone.0008349. PMID: 20041135; PMCID: PMC2796387.
Tillery EE, Ellis KD, Threatt TB, Reyes HA, Plummer CS, Barney LR. The use of the ketogenic diet in the treatment of psychiatric disorders. Ment Health Clin. 2021;11(3):211-219. Published 2021 May 12. doi:10.9740/mhc.2021.05.211.
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015 Mar;21(3):263-9. doi: 10.1038/nm.3804. Epub 2015 Feb 16. PMID: 25686106; PMCID: PMC4352123.