
By his friend William Curtis
Sometimes it takes a prediction coming true to prove a hypothesis. Einstein predicted the gravity of the sun would bend the light from stars. He had to wait for Edington to take pictures of stars in the background of the sun. These stars are normally hidden by the sun’s light. A shift in position of those stars would indicate that those beams of light were bent as they raced past the nearby sun into Edington’s camera during the 1919 total eclipse demonstrating Einstein’s prediction and changing the scientific paradigm. Dr. Veech made a bold hypothesis in several papers in 2002 and 2003 that ketones would be important in medicine. An examination of his life’s work leads one to ask, “Is there evidence of these predictions? Are ketones becoming important in medicine?”
Photo Copywrite by the Yu Foundation Used by permission.
His friends and colleagues called him “Bud”. Everyone who worked in his lab, Martin Kemper, Robert Pawlosky, Todd King, Calvin (Crunch) Crutchfield, Yoshihiro (Kashi) Kashiwaya and many, many others called him Dr. Veech out of respect. Those who worked closest with him held him in highest esteem. Perhaps this was because Dr. Veech understood biochemistry from a different perspective, one that was not taught in books. Rather one that came from having one’s work guided by annual reviews and direction from Hans Krebs, Albert Lehninger and others of that stature.
In addition to asking if ketones have become important to medicine, a second objective is to provide the reader with a framework to understand how Dr. Veech could have insights not available to those who did not fully grasp the impact of thermodynamics on the “great controlling nucleotide enzymes” or the impact of thermodynamics on the distribution of the nine Krebs-Henseleit ions. These topics are missing in textbooks. The understanding of the great nucleotide coenzymes allowed a paradigm shift from the old paradigm summarized in this statement: “Ketones are the poison found in ketone bodies that cause deadly diabetic ketoacidosis”. The current paradigm is one expressed by George Cahill and Richard Veech in the title of their 2003 hypothesis paper, “Ketoacids? Good Medicine?”.
Two other potential paradigm shifts are implied by Dr. Veech’s understanding of thermodynamics. One deals with a potential revolution in metabolomics. which Dr. Veech referred to in their current state as “metabaloney”. The third potential scientific revolution is based on the thermodynamics of the nine Krebs-Henseleit ions and a future impact on intravenous fluids. I hope to write an explanation of each of those topics in separate essays.
George Cahill was his mentor at Harvard medical school. Dr. Veech credits George Cahill for laying the foundation for his study of ketones. After getting his MD, Dr. Veech studied biochemistry with Hans Krebs, one of the greatest biochemists of the century.
Krebs is most widely known for putting the final steps together in the uric acid and the tricarboxylic acid cycles. These cycles can be found in wall charts known as metabolic pathways. Krebs and Veech did not study metabolic pathways. They turned their attention instead to thermodynamics. The equations of Josiah Willard Gibbs of Yale made it possible to study thermodynamics by freezing tissue and then measuring concentrations of biomolecules.
The great controlling nucleotide coenzymes
Metabolic pathways plot the conversion of one biochemical to another. This happens as biomolecules are built up to participate in the normal function of cells. Other times biomolecules are broken down into simpler molecules to extract energy or to make a waste product that can be excreted. Dr. Veech had a map of the metabolic pathways that covered a wall in his home office. The two circular pathways of Krebs stand out. All along these pathways one finds compounds entering and leaving the path. One sees the same three to five letter abbreviations for coenzymes appear repeatedly. They seem to come out of nowhere on the path. They are converted to a slightly different set of letters. Not only do the coenzymes change in the reactions, the biochemicals on the main path are changed as well in each reaction. For example, ATP is converted to ADP or vice versa. Sometimes the letter changes are in one direction, sometimes in the other, and sometimes in both directions. NAD converts back and forth to NADH. NADP converts to NADPH. CoA appears to be converted to many forms. Dr. Veech called the coenzymes represented by these letters the ”great controlling nucleotide coenzymes”.
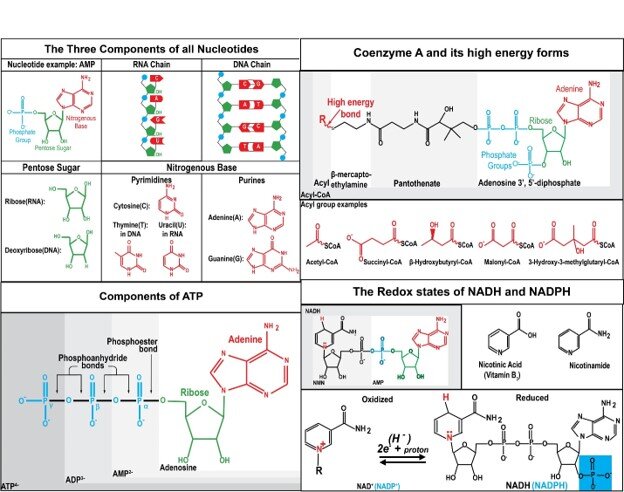
Measuring the potential energy of the great controlling nucleotide coenzymes
Figure 1. The great nucleotide coenzymes. illustration by Sam Lally, grandson of Richard L. Veech.
Although pathways are important, it is just as important to know what role is played by these 3 to 5 letter abbreviations of coenzymes that magically appear over and over along those paths. These great nucleotide coenzymes work like a group of circus acrobats jumping from a platform onto a teeter board. The acrobats convert potential energy to kinetic energy when they jump down. Standing on the downside of the teeter board is an acrobat that will be hurled spinning into the air and land in a seat. The coenzymes provide chemical potential energy needed to drive the metabolic pathways. Acrobats must carefully measure the heights and mass and know the formula for the conversion of potential energy to kinetic energy. Potential energy of the launching acrobats that jump from a platform determines if a stunt is likely to succeed. In the same way the potential energy of the great controlling nucleotides determines the likelihood of all the chemical reactions in the metabolic pathways.
Josiah Willard Gibbs provided the thermodynamic equation describing chemical potential. It is known as the Gibbs free energy equation. It is a measure of the energy found in the chemical bonds and a measure of energy related to changes in entropy. Apparently, it takes energy to organize things. Dr. Veech would use this equation along with a close variant proposed by Walther Nernst.
Why two equations?
Gibbs free energy does not account for transfer of electrons. In addition to bond energy and entropy, NADH and NADPH also transfer electrons. The reactions that transfer electrons are called oxidation and reduction reactions. The short term for oxidation and reduction is redox reactions. The kind of potential energy used to measure redox reactions using the Nernst equation is called the redox potential. With these equations, Dr. Veech explored the chemical and redox potentials of the nucleotide coenzymes that populate every region of the metabolic pathway chart. See Figure 2.
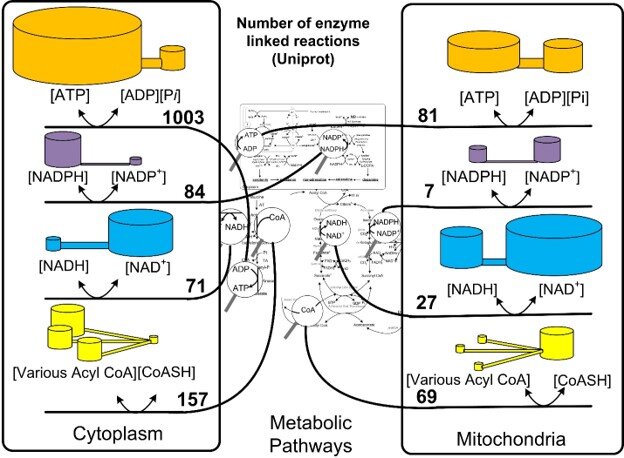
Figure 2 The great controlling nucleotides in their compartments. The magnifying glasses in the center show examples of reactions where the coenzymes are found in the metabolic pathways. The number of enzymes that share the potential energy in the same compartment are shown in the numbers. The two cylinders for each coenzyme couple are meant to show that the high energy and low energy forms have different concentrations and the ratio determines the potential energy.
Dr. Veech found the potential energies were different in the cell’s energy producing organelles, the mitochondria, than they were in the cytosol. This greatly complicated their measurement. Dr. Veech would complain whenever he saw these potentials measured wrong. To ignore the proper way of measuring the potential energy is as useful and as wrong as trying to weigh the led in a battery to determine its voltage. This happened often. One can find this mistake published in the leading journals. Because the potential energies are different in the mitochondria and the cytosol, one cannot combine the two and get meaningful information.
βHB could provide more energy than other energy molecules
Dr. Veech measured these potential energies in the liver, the heart and the brain in both fasted and fed states. His discoveries led him to understand that one of the ketone bodies, βHB, had special properties as a fuel. Ketone bodies are small water-soluble energy molecules that fuel the brain, the heart and other organs when we are not continuously consuming carbohydrates or protein.
Dr. Veech found out βHB could provide more energy than other energy molecules. This was recently demonstrated at the 2019 Tour de France. Each of the top 3 cyclists ascending the podium for the general classification category was known to be taking ketone monoester, an exogenous source of βHB called ΔG®. It was developed by Dr. Veech and his long time Oxford collaborator Prof. Kieran Clarke.
Dr. Veech did not set out to have his work used for athletes to win medals. His heart was in helping people. He wanted the ketone monoester to be used in medicine.
βHB can overcome a lack of energy found when a gate prevents pyruvate from fueling the mitochondria
Dr. Veech knew that many diseases share a common trait. That trait is that in damaged, or stressed cells the gate that lets the glucose breakdown product, pyruvate, into the mitochondria shuts a door making it harder for pyruvate to enter. He also knew βHB provided a way to circumvent the gate that blocks pyruvate.
The gate: Pyruvate Dehydrogenase Complex
In order to understand the importance of the gate one needs to know a few things about this gate. The gate is a collection of closely linked enzymes known as a complex. The complex acts on pyruvate and it removes a hydrogen. Hence the name pyruvate dehydrogenase complex or PDC. Inside the PDC, an enzyme working with the coenzyme NAD (hence the name coenzyme) transfers the hydrogen from pyruvate to NAD to make it NADH. We already know that NAD and NADH are among the great controlling nucleotides. We also know that both NADH and NADPH transfer electrons in addition to making or breaking chemical bonds and changes in entropy. We also know the potential energy available from NADH and NADPH is measured in redox potential. The energy from pyruvate is transferred with the electrons from pyruvate to NAD making it NADH. The careful reader may object. How can hydrogen carry electrons?
How can a hydrogen transfer an electron?
Hydrogen consists of one electron and one proton. It has no charge. The actual transfer from pyruvate to NAD+ is 2 electrons and one proton. This transfer of two electrons and one proton is equal to a hydride ion or H- being transferred. The reaction of pyruvate with NAD to make NADH is a redox reaction in the metabolic pathway. It also is one that collects energy from food and stores it in one of the great controlling nucleotides. This stored energy in NADH will be used to drive other reactions. Each NADH made in the mitochondria eventually creates 3 ATP.
What else does the PDC make?
The multiple enzymes in the PDC also split one carbon off the three carbon pyruvate to produce a molecule of CO2 . The energy from breaking this bond is used to attach coenzyme A to the last two remaining carbons. This makes acetyl CoA. The acetyl CoA makes 12 ATP in the mitochondria as it goes through the Krebs cycle.
To review, we learned that the pyruvate dehydrogenase complex or PDC functions as a gate. When it is open, it allows the transfer of an electron from pyruvate to NAD to make NADH. NAD and NADH work together in metabolic pathways. NAD collected energy from pyruvate to become NADH. The high energy form, which is NADH, is then available to be turned back into NAD passing its energy to another step in the metabolic pathway.
What closes or opens the gate that lets pyruvate into the mitochondria energy process?
Four different enzymes start to close the pyruvate dehydrogenase complex gate and two enzymes can open it once partly closed. These enzymes regulate the opening and closing of the gate to let pyruvate into the mitochondria. The control of these enzymes is complex, but we know the gate is often closed in degenerative disease, infections, and trauma.
Dr. Veech knew βHB does not go through the PDC to fuel the mitochondria. There are no gates to stop βHB from making ATP once it reaches the mitochondria. This means βHB can overcome a lack of energy found when the PDC switch turns off the flow of pyruvate fueling the mitochondria.
Dr. Veech’s mentor at Harvard, George Cahill, had demonstrated that the brain could use βHB for fuel when a person is fasting. Dr. Veech reasoned that βHB could help people who have diseases where the gate that lets pyruvate into the mitochondria has been shut down. This includes many degenerative diseases such as Alzheimer’s and Parkinson’s disease.
βHB can provide the energy to make neurotransmitters
Dr. Veech knew from his visiting scientist, Kashi, that NADPH was needed to synthesize several neurotransmitters that have low concentrations in Parkinson’s disease. The pathway to make a neurotransmitter involves transferring electrons from βHB. The electrons follow several steps along the metabolic pathway, the electrons go from βHB to NADPH to tetrahydrobiopterin, a 4 (tetra) hydrogen (hydro) version of biopterin. Next the electrons are transferred to the amino acid tyrosine. This pathway results in the change of tyrosine to L-DOPA.
Recall that L-DOPA is the precursor of dopamine. So, Dr. Veech predicted βHB would raise the levels of neurotransmitters involved with Parkinson’s. This was the predicted result based on the redox potentials of βHB then NADPH then tetrahydrobiopterin. Dr. Veech saw the data for dopamine increase, in the results of Dr. David Lovinger’s group. They showed that in a mouse model of Parkinson’s disease the ketone ester was able to restore the synthesis of dopamine. The data is yet to be peer reviewed or published, but the results encouraged Dr. Veech.
Professor Kieran Clarke is running studies using their ketone ester drink, ΔG®, to treat people with Parkinson’s.
Dr. Mark Hallett and Dr. Debra Ehrlich and Dr. Codrin Lungu have been involved in moving ketosis to a clinical trial at NINDS. Dr. Alexander Choi is planning on doing the necessary preliminary studies needed to understand ketosis in Parkinson’s. One day this may lead to a trial on overcoming the problem seen in Oliver Sack’s book “Awakenings”. The problem was that the L-DOPA therapy that had caused the awakenings stopped working. The book and movie “Awakenings” are about the use of L-DOPA in encephalitis lethargica and not Parkinson’s disease. However, dopamine therapies also stop working in people with Parkinson’s. One day it may be possible to study to see if ketosis can rescue patients resistant to L-DOPA therapy.
Can ketone monoester protect from lethal doses of radiation?
Dr. Veech reasoned that BHB’s effect on NADPH could overcome free radical damage. His first assignment when studying the great controlling nucleotides was to determine the NADP/NADPH redox potential. He had seen from the very first published measurements that somehow the redox couple was greater at reducing (had a more negative voltage or more H- to donate) in starved rat liver than in well fed rat liver. Bud knew the BHB produced in fasting could increase the reducing power of NADPH by making a larger percentage of the pool of NADP and NADPH into NADPH. The least complicated source of free radical damage is perhaps ionizing radiation. The hypothesis that BHB in the form of ketone monoester, ΔG®, could overcome ionizing radiation had been demonstrated to Dr. Veech before his death by Dr. Alexandra Miller’s work at the Armed Forces Radiobiology Research Institute. Although unpublished and yet to be peer reviewed, Dr. Veech was able to see the ketone monoester overcome a lethal dose of radiation in mice.
Where does the list end?
Soldiers are being studied for the effect of ketones on traumatic brain injury. Others are looking into ketosis or exogenous ketones for stroke, heart disease and diabetes. The reader is perhaps starting to think these claims are starting to go beyond science. Red flags are going up. Will the author next tell me that it works like statins, (hopefully without the bad side effects) and may treat erectile dysfunction? If Dr. Veech were writing, he would most definitely say yes it does work in all these ways and that he had seen this in animal studies and a few human subjects.
What is a realistic expectation?
Let me temper the enthusiasm of any who believe that one could magically cure any of these diseases with metabolic therapy. In the case of Parkinson’s disease which the author has had for twenty years, the ketone ester (commercially obtained) by itself did nothing. One must be in a state of ketosis for the ketone ester to have an additive effect. This is an anecdote, a N of one experiment with no investigational review board approval, but it is straight forward to tell when something has no effect.
The diagram below shows some of the differences between the fasted and the fed state. It demonstrates the complexity of the two states. The ketone ester works in my Parkinson’s when I am in the fasted state.
Taking exogenous ketones can put the body into states that were not possible to achieve before the ketone food supplements became available. We know very little about this third state that can allow one to be in the fed state and still have high levels of βHB. In the diagram βHB is called by its full name (R)-3-hydroxybutyrate.
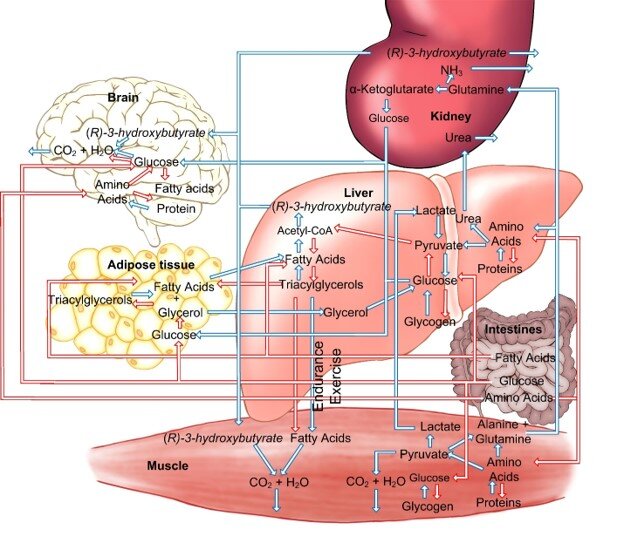
Figure 3. Blue arrows indicate pathways in the fasted state. Red arrows indicate pathways in the fed state. (Illustration by David Lamps and William Curtis)
We are still a long way from fully understanding metabolic therapy. A rapidly expanding group of scientists are now starting to understand what Dr. Veech found when he and Krebs started studying the thermodynamics of the great controlling nucleotide coenzymes. Dr. V. Bohr (the grandson of the physicist Niels Bohr) at the National Institute on Aging, is looking at the effect of combining βHB with nicotinamide riboside and has produced interesting results in animal models of Alzheimer’s disease in collaboration with Dr. Veech’s group at NIAAA, a division of NIH devoted to alcoholism.
Synergism of ketosis and nicotinamide riboside food supplements
In some of the Alzheimer’s experiments it appears that the βHB is needed for nicotinamide riboside to have any results. In other words, unless the βHB makes NADPH available to provide electrons, eating the nicotinamide riboside precursor of NADPH has no effect, but when the precursor is added to βHB the results are synergistic. This can be easily explained if one understands the math behind the redox potential. Both the Nernst redox potential and the Gibbs free energy derived equations Dr. Veech used had terms where the concentration of products over the concentration of the reactants were used to determine the redox potential. In this equation the concentration of the pair of nucleotides cancels out. That means the redox potential at any given steady state or near equilibrium is only proportional to the ratio of [NADP] to [NADPH]. It is not affected by the number of the total of NADP plus NADPH. In fact, when Dr. Veech or anyone else measured the Gibbs free energy or the redox potential of the great controlling nucleotides, they only had to calculate the ratio of the low energy form to the high energy form.
Can vitamins and antioxidants do the same thing as βHB?
One may ask, how would redox potential be affected if precursors of NADPH are added by way of the diet so that there was more total NADPH plus NADP? From the data that shows under certain conditions NADP precursors have no effect on their own in the absence of βHB making the redox potential more reducing (likely to transfer an electron), one can infer that antioxidants and supplements may not work on their own. In the case of nicotinamide ribosides, precursors of NADH and NADPH the effect of having more total purine nucleotides is like having a larger battery, one with more cold cranking power, but the same voltage. The precursor supplement does not change the redox potential unless NADPH utilization goes up. One could expect similar behavior as that found in a battery. There is no difference in the voltage of a 1.5 volt triple A battery and a 1.5 Volt D Battery. Using a volt-meter, one cannot tell a triple A battery from a D battery. If you put both batteries under load, the smaller AAA battery voltage drops faster than the D battery under the same load of current draw. Likewise, If the NADPH has a small load the voltage or redox potential stays the same. It is when one puts a load on the battery or starts to use NADPH rapidly that the voltage drop is greater in the small AAA battery than in the larger D battery. Also, the redox potential or ability to provide electrons is reduced faster when there is a demand for NADPH and the total concentration is lower.
To summarize this somewhat complicated discussion, βHB is the source of energy that can produce NADPH from NADP when the glucose pathway is not able to produce NADPH. In situations where there is a load on NADPH such as during fatty acid synthesis, or when there is oxidative stress or inflammation shutting the PDC gate, then I think vitamin precursors of the great controlling nucleotides and antioxidants may have a synergistic effect when used in combination with βHB.
What does the future hold?
The study of synergies offers great hope that the therapies will work, given time and further research into other synergies. Perhaps synergies with ketosis will even work with cancer. There are many fields where scientists are beginning to comprehend that metabolic interventions may be needed to tackle treatment of the disease they study. The current thinking is that several interventions will be needed to suppress the cancer cells.
In Dr. Veech’s lab, Dr. Robert Pawlosky, and Todd King, are busy making the measurements of potential energies of the great nucleotide coenzymes and producing ketone ester for animal studies for studies in Parkinson’s, Alzheimer’s, heart disease and radiation mitigation to name the major collaborations.
When did Dr. Veech have time to study alcoholism?
NIH is divided up by disease constituents. There is no disease for basic research. Dr. Veech is part of the division that serves alcoholics, the NIAAA. Dr. Veech was grateful that the NIAAA gave him a home at NIH, but he did not study things he could have studied regarding the mission of NIAAA. Serendipitously, the ketone monoester is a candidate for treatment of alcohol addiction as well as fatty liver disease. Both fall under the mandate of NIAAA. Dr. Corinde Wiers has proposed studying the use of the ketone ester for the purpose of breaking the craving for alcohol.
Dr. Veech died at the age of 84 after giving a presentation to the Keystone Symposium on Diabetes on Wednesday January 29th. On Thursday morning he flew home to his modest home in Rockville. He passed away shortly after walking into his home and sitting down. He had been to the mountain. He had seen the applications that resulted from the basic research. His prediction along with Cahill of “Ketoacids? Good Medicine?” is being tested. Metabolic therapy has captured the attention of the medical community. The expected promise to change lives that drove Richard Veech to keep working to the age of 84 will require the work of those who understand what Richard Veech understood. It is my hope in writing this article that you would now understand the basis for his predictions and that you would become a champion for the studies and trials needed to prove or disprove his hypothesis that ketones would make good medicines.
Written by: William Curtis
