Epilepsy, neurodegenerative diseases, cancer, and beyond – research into the therapeutic applications of nutritional ketosis continue to expand. In fact, our team recently published a review on the anticatabolic effect of ketone bodies in skeletal muscle [1].
Muscle loss is the result of an imbalance between muscle protein synthesis and muscle protein breakdown, with the latter dominating.
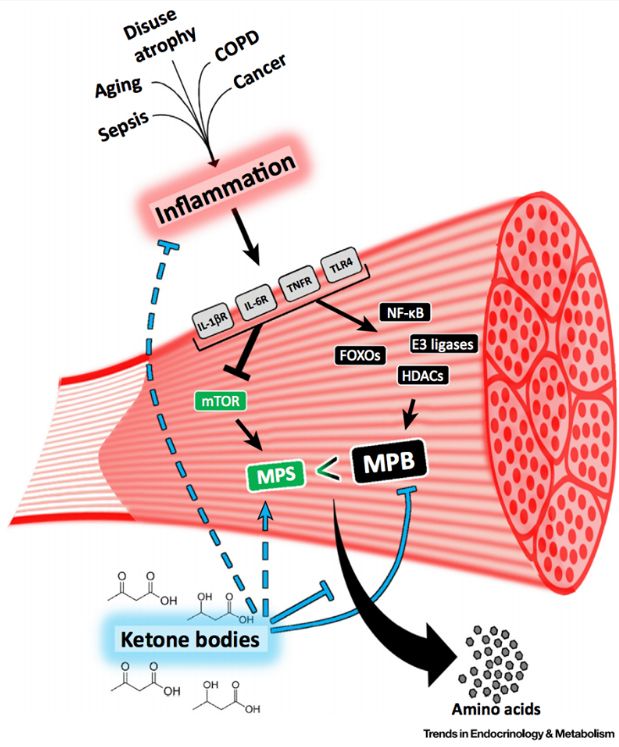
Loss of muscle mass and muscle function is stated by many as a “natural part of aging”, and sadly it is a strong predictor of mortality. Muscle wasting is not only associated with age, but it is also a devastating side effect of many diseases and conditions, including the ability to receive and respond to standard of care therapies – most of which is strongly influenced by inflammation. Despite being a major contributor to poor clinical outcomes, there are currently no effective strategies to mitigate muscle wasting.
How could ketones prevent muscle wasting?
Through the evolutionary lens, it makes sense that ketones offer protection against breaking down our muscle to convert certain amino acids to glucose (aka gluconeogenesis). When we are in a state of nutritional- or starvation-ketosis, we have undergone a transition where glucose utilization slows down and our metabolism has switched to using alternative fuel sources, such as free fatty acids and ketones. If we weren’t able to make ketones from fat we would quickly wither away during times of food deprivation. This explains the muscle-sparing effect of ketosis. In the keto-adapted individual, free fatty acids are the primary fuel for muscle tissue, sparing ketones to other tissues (e.g. brain and heart).
In this particular review paper, inflammation-mediated muscle wasting is highlighted, as it is known that inflammation and oxidative stress pathologically underpin and drive muscle wasting. It is also known that ketone bodies are potent anti-inflammatory molecules and can reduce oxidative stress. The research suggests that ketone bodies, particularly beta-hydroxybutyrate (BHB), could be preventing inflammation-driven catabolic pathways.
Evidence suggesting that ketones are anticatabolic:
· Experimentally, an elevation of plasma ketones reduced muscle protein turnover in catabolic scenarios, such as surgical recovery, skeletal trauma, sepsis, and severe burns [2]
· Ketones have even been shown to promote muscle protein synthesis in humans and animal models [3, 4]
· In a study of inflammation-induced muscle atrophy, the ketone body, BHB, reduced the marker of muscle breakdown used in this experiment (phenylalanine efflux from muscle) by 70%. They even compared the effects with hyperinsulinemia and BHB was a more potent anticatabolic stimulus [5]
Ketones may just be something that can shine in real-life situations such as sarcopenia, disuse, and disease-related atrophy, such as cancer cachexia. Therapeutic ketosis, whether induced with a ketogenic diet or exogenous ketones, is safe and tolerable. Not to mention that ketones offer a host of other benefits outside of their muscle sparing effects.
We are excited to see where the research is heading because we do believe ketones could have major implications as a mitigation strategy for the sad reality of muscle wasting.
Written by: Kristi Storoschuk; Edited by: Dr. Dominic DAgostino
Resources:
1. Koutnik, A.P. et al. (2019) Anticatabolic Effects of Ketone Bodies in Skeletal Muscle. Trends Endocrinol Metab. 30(4), 227-229.
2. Thompson, J.R. and Wu, G. (1991) The effect of ketone bodies on nitrogen metabolism in skeletal muscle. Comp. Biochem. Physiol. B 100, 209–216
3. Evans, M. et al. (2017) Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J. Physiol. 595, 2857–2871
4. Nair, K.S. et al. (1988) Effect of beta-hydroxybutyrate on whole- body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J. Clin. Invest. 82, 198–205
5. Thomsen, H.H. et al. (2018) Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: anticatabolic impact of ketone bodies. Am. J. Clin. Nutr. 108, 857–867
