The gut microbiome has gained a lot of attention over the last decade or so, with more and more research suggesting a fundamental role in health and disease – so much so, some scientists even refer to it as “the last human organ”.
As far as we know, there is no “optimal” microbiome, and there is certainly a great deal more to be discovered. With that said, there appears to be some characteristics associated with both a “healthy” and “unhealthy” gut microbiome, the latter of which has been associated with various ailments, diseases, and even mental illnesses. It’s well agreed upon that what you eat influences the gut microbiome, and thus exploring the effect of different dietary factors, such as the ketogenic diet, is an emerging area of interest.
What is the gut microbiome?
Humans are home to an entire ecosystem of microorganisms, collectively known as the gut microbiota, consisting of trillions of microbial cells including thousands of different bacterial species that colonize the gastrointestinal tract. The gut microbiome, on the other hand, encompasses the genes and functions of these species (aka the interactions between these critters and the host – us). The most prominent bacterial species in the human gut microbiota belong to the Bacteroidetes and Firmicutes phyla.
What is the role of the microbiome?
The gut microbiome serves various fundamental roles in humans. In return for providing them with a nurturing environment, they make sure to keep us happy and healthy. However, the opposite holds true, too, where exposing the gut to environmental factors that promote dysbiosis can end up harming us as the host. The gut microbiota has been shown to influence obesity, inflammation, immunity, digestive disorders, auto-immune disorders, and more. For example, reduced levels of Bacteroidetes and increased levels of Firmicutes are found in those with irritable bowel syndrome (IBS), obesity, type 2 diabetes, and are associated with altered blood glucose metabolism, compared to healthy individuals.
Example roles of the gut microbiome
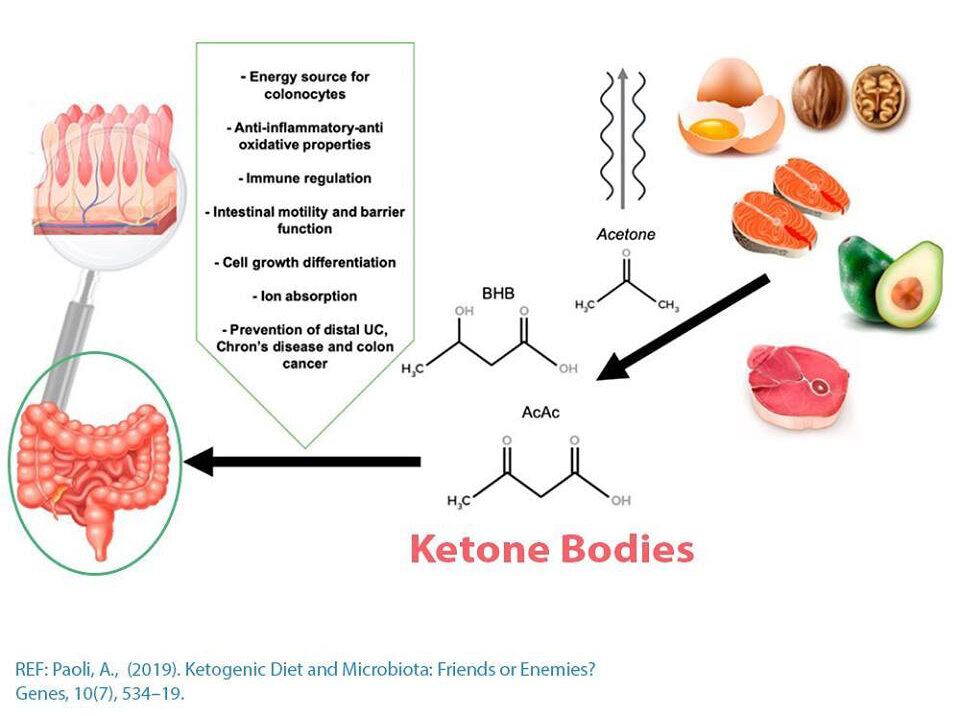
· The gut microbiota maintains the integrity of our gut lining through the production of short-chain fatty acids (SCFAs) that act as the major source of energy for our intestinal cells (coloncytes), in addition to promoting intestinal epithelial cell turnover (regeneration of new cells). A healthy gut lining and therefore maintenance of normal gut permeability plays a crucial role in preventing pro-inflammatory molecules, foreign proteins, toxins, etc. from getting into circulation and triggering an immune response. Gut permeability is also referred to as the more commonly known “leaky gut”, and therefore a function of the gut microbiome is to prevent this from occurring.
· The gut microbiota synthesizes and metabolizes certain nutrients, essential vitamins (e.g. vitamin K and B vitamins), and hormones.
· The gut microbiota defends against pathogens (e.g. viruses, pathogenic bacteria, etc.) by out-competing for nutrients, in addition to secreting secondary metabolites that help fight against pathogens.
· The gut microbiota converts undigested food matter (e.g. dietary fiber) into usable energy and metabolites, in the large intestine where the majority of the gut microbiota resides.
· The gut microbiome communicates with the brain through the nervous system, endocrine system, immune system, and metabolic system in a bidirectional communication, otherwise known as the gut-brain-axis. For example, certain gut bacteria synthesize neurotransmitters and neural regulators that act as signaling molecules between the gut and the brain.
· The gut microbiota modulates immune function through the various roles listed above (e.g. maintaining the integrity of the gut lining, protection against pathogens, and gut-brain-axis). A healthy gut microbiome, therefore promotes a healthy immune system, prevents inflammation, and may protect us from disease.
What effect does the ketogenic diet have?
Because this field is so new, what we know about the ketogenic diet and the microbiome is limited to a small set of studies, animal models, and on top of that have produced mixed results. Also, it’s really not as simple as saying the ketogenic diet does “X” to the microbiome, because everyone’s ketogenic diet will look a bit different, and the quality/composition of the diet matters.
A common observation, however, is a decrease in the number of species types (aka diversity), which makes sense given that the ketogenic diet limits an entire macronutrient (carbohydrates). Different microbes feed off different food sources, so restricting carbohydrates to the extent of a ketogenic diet can starve off certain “carbohydrate-loving” species. Worth noting is that although low gut microbiota diversity has been linked to worse health outcomes, these associations are made largely in the context of a mixed diet. It’s unknown whether this decrease in diversity would have any long-term health implications in the context of a ketogenic diet.
In a recent review by Paoli et al., they summarize some of the research pertaining to how the ketogenic diet may alter the gut microbiome. Here is what they found:
– In a mouse model of epilepsy, the ketogenic diet significantly increased two species of bacteria Akkermansia and Parabacteroides, both shown to be essential for the anti-seizure effect of the diet. These changes to the gut microbiome ultimately increased the brain’s GABA (calming neurotransmitter) to glutamate (excitatory neurotransmitter) ratio, a mechanism in which the ketogenic diet elicits its anti-seizure effects.
– A ketogenic diet composed of all three types of fats: saturated, monounsaturated, and polyunsaturated fatty acids, given to mice for sixteen weeks improved neurovascular function and lowered risk of Alzheimer’s disease. Analysis of the gut microiome showed an increase in the beneficial SCFA-producing bacteria, Akkermansia Muciniphila and Lactobacillus, a reduction in the inflammation-promoting bacteria Desulfovibrio and Turicibacter, and a decrease in diversity due to the low-carbohydrate content of the diet. The authors suggest that the changes to the gut microbiome may be related to the improvements in neurovascular function.
– In an animal model of autism, ten to fourteen days on the ketogenic diet improved the ratio of firmicutes to bacteroides which is typically out of balance in autism spectrum disorder, and also related to various other metabolic conditions. This improvement was associated with improved behavioral symptoms. In addition, the ketogenic diet brought levels of A. muciniphila down to normal levels, and the authors concluded an overall “anti-microbial” effect of the diet.
– In patients with multiple sclerosis (MS), the ketogenic diet restored the biofermentative function of the gut microbiome, which is commonly impaired in MS. Microbiota fermentation allows for the production of beneficial SCFAs, and impairments to this function can promote the production of potentially harmful compounds. Diversity initially dropped significantly but returned to baseline within twelve weeks, and exceeded baseline conditions by weeks twenty-three and twenty-four.
– In children with epilepsy, the ketogenic diet improved imbalances in the gut microbiome, present prior to starting the intervention. Specifically, the diet significantly decreased pathogenic bacterial species, and increased Bacteroidetes, both in the patients and the healthy control group. Bacteroides species are thought to be connected to the anti-seizure effects of the diet as they secrete molecules involved in the gut-brain-axis.
– In a population of pediatric epilepsy, the ketogenic diet improved the gut microbiome (increased Bacteroides and decreased Firmicutes and Actinobacteria) in those that “responded” to the diet, i.e. seizure frequency was reduced or stopped. These changes were not observed in the non-responders, and these individuals showed an increase in Clostridia, Ruminococcus, and Lachnospiraceae (Firmicutes). These results suggest the gut microbiome is playing a role in the efficacy of the ketogenic diet against seizures.
– In patients with GLUT1 deficiency syndrome, the ketogenic diet was shown to increase a potentially harmful bacterial species, Desulfovibrio spp., a species that has been associated with inflammation of the gut.
– In epileptic children, the ketogenic diet reduced a subset of species that are associated with promoting health, Bifidobacteria, E. rectale, and Dialister.
What’s the takeaway?
Overall, the ketogenic diet and the presence of ketone bodies may support a healthy gut microbiome in healthy individuals and restore imbalances in those with certain conditions that respond to the ketogenic diet. Commonly observed is a decrease in diversity, which corresponds to the reduction of carbohydrates in the diet. There are mixed results for SCFA-production, and this is likely dependent on the composition of your ketogenic diet (i.e. inclusion of dietary fiber). The alterations in the gut microbiome likely play a role in the efficacy of the diet on a whole-body level. For example, an increase in SCFA and changes in enzymes involved in modulating the brain’s GABA to glutamate ratio. Certain species that are associated with weight loss and improved insulin sensitivity have been shown to increase on a ketogenic diet (e.g. A. muciniphila and Lactobacillus).
Here are some things to consider when formulating your ketogenic diet:
Fiber: Dietary fiber can support the growth of SCFA-producing bacteria, which as mentioned, feed our intestinal cells and have been shown to play a beneficial role in our immune system. Many low-carb vegetables contain the type of fiber (soluble) that promote the production of SCFAs. Results from ketogenic diet studies have produced mixed results – both increases and decreases SCFA production, likely corresponding to the amount of fiber (non-digestible carbohydrates) in the ketogenic diet used in the particular study. Various studies have shown an important role for fiber as a source of energy to gut microbes, and the benefits of their by-products. Therefore, making sure to consume low-carbohydrate fibrous vegetables may be a good idea to promote the growth of these biofermentative bacteria.
Fat: You may be aware of the harmful effects of the highly processed, industrial seed oils, otherwise referred to as “vegetable oils”. These oils are very high in omega-6 polyunsaturated fatty acids (PUFAs), which when over-consumed are associated with increased inflammation, and this may be related to their effect on the gut microbiome. Whereas, omega-3 PUFAs are associated with improvements in gut microbiota. Consuming vegetable oils such as soybean, corn, canola, cottonseed, sunflower, etc. can contribute to this imbalance in omega-6 to omega-3 and this can have negative consequences on the gut microbiome. It is important to consider the type and quality of fats you are consuming on a ketogenic diet. Some examples of high quality fat sources are: grass-fed meat, eggs, fatty fish, MCT oil, grass-fed butter/ghee, unrefined virgin coconut oil, avocado oil, extra-virgin olive oil, low omega-6 nuts/nut butters.
Dairy: In the past, the classical ketogenic diet relied heavily on full-fat dairy. However, many people suffer from lactose malabsorption whereby lactose, a carbohydrate found in milk, reaches the large intestine undigested where it is fermented by gut microbes. In some people, these fermentation products can cause discomfort. Interestingly, lactose fermentation has been shown to increase various SFCA-producing bacteria, a characteristic of a healthy gut microbiome. However, as mentioned, lactose malabsorption is typically accompanied by uncomfortable symptoms. What’s health promoting in one person, may not be in another, and typically if something in your diet makes you feel bad, it’s probably a telling sign not to eat it. This also highlights not only the infancy, but the complexity of understanding how the gut microbiome impacts our health. All in all, dairy consumption is highly dependent on the individual and there is a lot more to discover.
Fermented Foods: Fermented foods appear to improve the gut microbiome diversity and overall gut health, although even this seems to be inconclusive. Nonetheless, these foods can provide both pre- and probiotics which can feed the SCFA-producing bacteria and possibly enrich the diversity of the microbiota by providing already living bacteria (probiotics). This includes fermented vegetables for example, sauerkraut and kimchee, and fermented dairy products such as kefir, fermented cheeses, and sugar-free yogurts, all of which can be included on a ketogenic diet in amounts that allow you to personally sustain ketosis.
Conclusion
We are just starting to scratch the surface of the intricacies of the human gut microbiome, and the potential impact of the ketogenic diet. To add to this, the gut microbiome can also be impacted by gender, genetics, ethnicity, medications, and other environmental exposures.
The take-home message is that with the research we have presented here, the ketogenic diet appears to have an overall beneficial impact on the gut microbiome associated with improved gut lining, anti-inflammatory properties, improved immunity, and modulating the gut-brain-axis. The changes that occur may actually be in part responsible for the therapeutic benefits of the ketogenic diet, in addition to correcting microbiome imbalances associated with certain diseases. The ketogenic diet can produce different results depending on what you choose to eat and nutritional ketosis can be achieved in a variety of ways. We hope the suggestions above help in formulating a ketogenic diet that promotes a healthy gut microbiome, all while preventing any potential unwanted changes.
Written by: Kristi Storoschuk; Edited by: Dr. Dominic DAgostino, Dr. Csilla Ari Dagostino
